Answer:
(a) ![]()
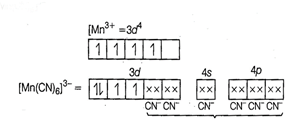 (i)
(i) ![]() hybridisation
(ii) Inner orbital complex because (n - 1) d-orbitals are
used.
(iii) Paramagnetic, as two unpaired electrons are present.
(iv) Spin only magnetic moment
hybridisation
(ii) Inner orbital complex because (n - 1) d-orbitals are
used.
(iii) Paramagnetic, as two unpaired electrons are present.
(iv) Spin only magnetic moment
![]() (b)
(b) ![]()
![]()
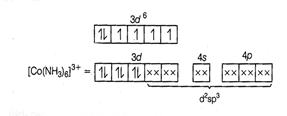 (
(![]() pair up the
unpaired 3d electrons.)
(i) d2sp3 hybridisation
(ii) Inner orbital complex because of the involvement of (n
-1) d-orbital in bonding,
(iii) Diamagnetic, as no unpaired electron is present.
(iv)
pair up the
unpaired 3d electrons.)
(i) d2sp3 hybridisation
(ii) Inner orbital complex because of the involvement of (n
-1) d-orbital in bonding,
(iii) Diamagnetic, as no unpaired electron is present.
(iv) ![]() (c)
(c) ![]()
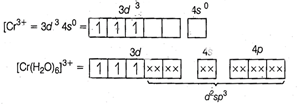 (i)
(i) ![]() hybridisation
(ii) loner orbital complex (as (n - 1)o'-orbital take
part.)
(iii) Paramagnetic (as three unpaired electrons are
present.)
(iv)
hybridisation
(ii) loner orbital complex (as (n - 1)o'-orbital take
part.)
(iii) Paramagnetic (as three unpaired electrons are
present.)
(iv) ![]() (d)
(d) ![]()
![]()
 (i)
(i)![]() hybridisation
(ii) Outer orbital complex because nd-orbitals are involved
in hybridisation.
(iii) Paramagnetic (because of the presence of four
unpaired electrons).
(iv)
hybridisation
(ii) Outer orbital complex because nd-orbitals are involved
in hybridisation.
(iii) Paramagnetic (because of the presence of four
unpaired electrons).
(iv) ![]()
You need to login to perform this action.
You will be redirected in
3 sec
