Answer:
(i) 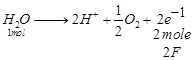 Quantity of electricity required
= 2F
(ii)
Quantity of electricity required
= 2F
(ii) ![]() Quantity of electricity required = 1F 1 x 96500 = 96500 C
Quantity of electricity required = 1F 1 x 96500 = 96500 C
You need to login to perform this action.
You will be redirected in
3 sec
