Answer:
From the Ellingham
diagram, we find that 983 K, the curves intersect.
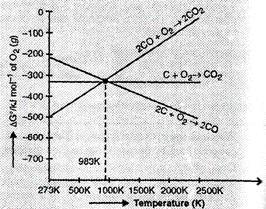 The value of
The value of ![]() G°
for change of C to CO2 is less than the value of
G°
for change of C to CO2 is less than the value of ![]() G°
for change of CO to CO2. Therefore, coke (C) is a better reducing
agent than CO at 983 K or above temperature. However below this temperature
(e.g., at 673 K), CO is more effective reducing agent than C.
G°
for change of CO to CO2. Therefore, coke (C) is a better reducing
agent than CO at 983 K or above temperature. However below this temperature
(e.g., at 673 K), CO is more effective reducing agent than C.
You need to login to perform this action.
You will be redirected in
3 sec
