Answer:
Free radical mechanism for the polymerization of
etheneinvolves following 3 steps.
1. Chain initiation step Phenyl
free radical formed by the homolysisof benzoyl peroxide adds to the double bond
of ethene. It results inthe formation of a new and larger free radical. This is
the chaininitiation step
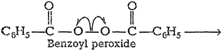
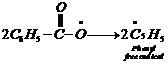
![]() 2. Chain propagating step
As this radical reacts with anothermolecule of ethene, another bigger sized
radical is formed. Thisprocess repeats continuously resulting in the
propagation of thereaction in the forward direction.
2. Chain propagating step
As this radical reacts with anothermolecule of ethene, another bigger sized
radical is formed. Thisprocess repeats continuously resulting in the
propagation of thereaction in the forward direction.
![]()
![]()
![]() 3. Chain terminating step
For termination of the long chain, thesefree radicals can combine in different
ways to form polythene. Thisis called chain terminating step.
3. Chain terminating step
For termination of the long chain, thesefree radicals can combine in different
ways to form polythene. Thisis called chain terminating step.
![]()
![]()
![]()
![]()
![]()
You need to login to perform this action.
You will be redirected in
3 sec
