Answer:
The
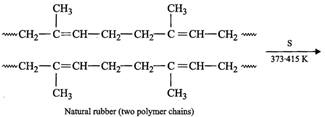
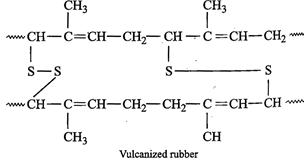
natural polymer of 2-methyl-l, 3-butadiene is cis-polyisoprene (natural
rubber). On heating with sulphur between 373-415 K, vulcanization occurs as a
result of which ?S?S? bridges or cross-links are introduced between polymer
chains through their reactive allylic positions as shown below :
You need to login to perform this action.
You will be redirected in
3 sec
