Answer:
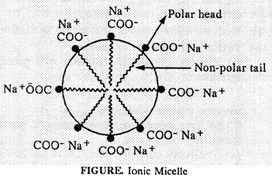
Soap is sodium salt
of higher fatty acids [RCOONa]. The nonpolar alkyl part of soap dissolves in
oil or greese while polar ![]() part dissolves in
water. Thus, each oil droplet is surrounded by soap aggregates and an emulsion
of oil in water is formed. This is called micelle. These oil droplets then get
detached from the cloth surface by rubbing and can be washed with water.
part dissolves in
water. Thus, each oil droplet is surrounded by soap aggregates and an emulsion
of oil in water is formed. This is called micelle. These oil droplets then get
detached from the cloth surface by rubbing and can be washed with water.
You need to login to perform this action.
You will be redirected in
3 sec
