Answer:
(c) Assertion is correct statement
but Reason is incorrect statement.
Assertion is true as in ccp atom
present at face centre and corner of each unit cell which creates octahedral
void at each body centre and all twelve edges of a unit cell as shown below
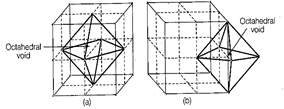 Correct reason is that beside
the body centre there is one octahedral void at centre of each of 12 edges
which is surrounded by six atoms.
Out of six atoms four belongs to
same unit cell (2 at corner and 2 at face centre) and 2 atoms belongs to
adjacent unit cell.
Correct reason is that beside
the body centre there is one octahedral void at centre of each of 12 edges
which is surrounded by six atoms.
Out of six atoms four belongs to
same unit cell (2 at corner and 2 at face centre) and 2 atoms belongs to
adjacent unit cell.
You need to login to perform this action.
You will be redirected in
3 sec
