Answer:
Cubic close packed structure contains one
atom at each of eight corners of a unit cell and
one atom at each of six faces which can
be represented below
As we know any atom surrounded
by six atoms (hard sphere) creates an octahedral void. In case of fee body
centre is surrounded by six identical atoms present at face centre hence, there
is a octahedral void at body centre of each unit cell.
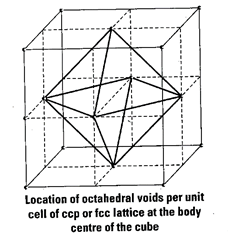 Beside the body centre there is
one octahedral void at centre of each of 12 edge as shown below
Beside the body centre there is
one octahedral void at centre of each of 12 edge as shown below
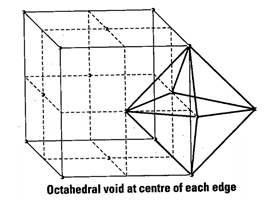 Since, each void is shared by 4 unit
cell. Therefore, contribution of octahedral void to each edge of a unit cell is
Since, each void is shared by 4 unit
cell. Therefore, contribution of octahedral void to each edge of a unit cell is
![]() .
Number of octahedral void at centre of
12 edge =
.
Number of octahedral void at centre of
12 edge = ![]() x 12 = 3
Number of octahedral void at
body centre = 1
Therefore, total number of
octahedral void at each ccp lattice = 3 +1 = 4
x 12 = 3
Number of octahedral void at
body centre = 1
Therefore, total number of
octahedral void at each ccp lattice = 3 +1 = 4
You need to login to perform this action.
You will be redirected in
3 sec
