Answer:
An
octahedral site is created by the arrangement of four spheres (atoms) in one
plane, one sphere (atom) on top and one sphere (atom) below this plane. The
shaded portion in the figure represent an octahedral void. For clarity, the
spheres present above and below the void are not shown.
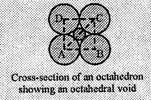 Let, length of each
side of the square is 'a' and the radii of the void and the sphere (atom) are r
and R respectively. Consider the right angled triangle ABC,
Let, length of each
side of the square is 'a' and the radii of the void and the sphere (atom) are r
and R respectively. Consider the right angled triangle ABC,
![]() As AC = 2r + 2R
As AC = 2r + 2R
![]() ?.(i)
Now AB = 2R or a =
2R
Dividing Eqn. (i)
by Eqn. (ii), we get
?.(i)
Now AB = 2R or a =
2R
Dividing Eqn. (i)
by Eqn. (ii), we get
![]()
![]()
![]() Thus, for an
octahedral void,
Thus, for an
octahedral void, ![]()
![]() For octahedral
void, the radius ratio = 0
For octahedral
void, the radius ratio = 0![]() 414
414
You need to login to perform this action.
You will be redirected in
3 sec
