Answer:
Semiconductor is
the solid which is perfect insulator at 0 K but conduct some electricity at
room temperature. e.g., Silicon and Germanium. Two main types of semiconductors
are n-type and p-type semiconductors.
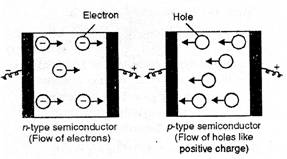
(i) n-type semiconductors.
Silicon and germanium (Group 14) have very low electrical conductivity in the
pure state.
If we add certain
elements like phosphorus (P) or arsenic (As) of group 15 to these covalent
crystals, their atoms will also get linked with those of group 14 elements by
covalent bonds but will have the extra electron which is not involved in the
bonding (atoms have five valence electrons). These extra electrons will lead to
electrical conductivity resulting in n-type semi conductors as these are
conducting due to the movement of electrons.
(ii) p-type
semiconductors. If in the covalent crystals of group 14 elements, the
addition of small amounts of the element aluminium (Al) or gallium (Ga) belonging
to group 13 is done, the atoms of such elements can share only three electron
with the atoms of group 14. Thus, the holes will be created in the lattice
since there is no fourth electron available for sharing. The holes will lead to
electrical conductivity and the crystals thus formed are also semiconductors.
These are known as p-type semiconductors.
You need to login to perform this action.
You will be redirected in
3 sec
