Answer:
(d) Hexagonal close packing can be arranging
by two layers A and B one over another which can be diagramatically represented
as
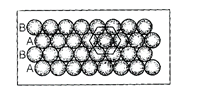 Here, we can see easily that 1st
layer and 4th layer are not exactly aligned.
Thus, statement (d) is not
correct while other statements (a), (b) and (c) are true.
Here, we can see easily that 1st
layer and 4th layer are not exactly aligned.
Thus, statement (d) is not
correct while other statements (a), (b) and (c) are true.
You need to login to perform this action.
You will be redirected in
3 sec
