Chemical Reations and Acids, Bases and Salts
Category : 10th Class
Chemical Reactions and Acids, Bases and Salts
Chemical Reactions
Its a process in which one or more substances/ the reactants are converted to one or more different substances, the products. During a chemical reaction, rearrangement of reacting substances takes place to form new substances, which have different properties than the original one. In a chemical reaction, substances are divided into reactants and products. Reactants are the substances that take part in a chemical reaction whereas products are the substances that are formed as a result of chemical reaction.
Endothermic and Exothermic Processes
In all the chemical reactions, transformation or change in energy is involved. On the basis of change in energy all the reactions are divided into two parts that are endothermic and exothermic reactions. The reaction in which heat is absorbed is called endothermic reaction. The reaction in which energy is given out in the surroundings is called exothermic reaction.
Photosynthesis is an example of an endothermic reaction. In the process of photosynthesis, plants by utilizing the energy of the sun convert carbon dioxide water and glucose and oxygen.
\[6C{{O}_{2}}+6{{H}_{2}}O\,\xrightarrow[Chlorophyll]{Light}\,{{C}_{6}}{{H}_{12}}{{O}_{6}}+6{{O}_{2}}\]
(Carbon dioxide) (Carbohydrate)
Sodium and chlorine are mixed together to yield table salt is an example of exothermic reaction. 411 kJ of energy is produced in this reaction.
\[Na+0.5C{{l}_{2}}(g)\to NaCl(s)+411\,\,KJ\]
Chemical Equation
A chemical equation is a way of writing or describing chemical reactions. It tells us that what happens when a chemical reaction takes place. It consists of information about reactants, products, the formulas of the reactants and products, the states of the reactants and products such as solid, liquid, gas and the amount of each substance.
Balancing the Equation
To get the same number of atoms of every element on each side of the equation, apply the law of conservation of mass. Now balance an element that appears only once in reactant and product. After balancing one element, proceed further to balance the next and continue balancing until all the elements are balanced. Now you need to balance chemical formulas by placing coefficients in front of them. Do not add subscripts because this will change the formulas.
Types of Chemical Reactions
All the chemical reactions are divided into six categories. These six categories are as follows:
Combination Reaction
When two or more elements or compounds combine to form a compound, then combination reaction takes place. They are mostly exothermic.
The combination of iron and sulphur to form iron sulphide is an example of combination reaction:
\[8Fe+{{S}_{8}}\xrightarrow{{}}8FeS\]
(Iron) (Sulphur) (Iron sulphide)
Decomposition Reaction
When a complex compound breaks down to make simple molecule, decomposition reaction takes place. They are always endothermic.
The electrolysis of water to make oxygen and hydrogen gas is an example of decomposition reaction:
\[2{{H}_{2}}O\xrightarrow{{}}2{{H}_{2}}+{{O}_{2}}\]
(Water) (Hydrogen) (Oxygen)
Single Displacement Reaction
When in a reaction an atom or a group of atoms present in a molecule is displaced by another atom, is known as displacement reaction. The displacement of silver when copper is added to a solution of silver nitrate is an example of displacement reaction:
\[Cu+2AgN{{O}_{3}}\xrightarrow{{}}Cu{{(N{{O}_{3}})}_{2}}+2Ag\]
(Copper) (Silver nitrate) (Copper nitrate) (Silver)
Double Displacement Reaction
In a chemical reaction when two compounds exchange their ions to form two new compounds, double displacement reaction takes place. Formation of copper sulphide and sulphuric acid when hydrogen sulphide gas is passed through a solution of copper sulphate is an example of double displacement reaction.
\[\underset{(Copper\,\,sulphate)}{\mathop{CuS{{O}_{4}}}}\,+\underset{(hydrogen\,\,sulphide)}{\mathop{{{H}_{2}}S}}\,\xrightarrow{{}}\underset{(copper\,\,sulphide)}{\mathop{CuS}}\,+\underset{(sulphuric\,\,acid)}{\mathop{{{H}_{2}}S{{O}_{4}}}}\,\]
Neutralisation Reaction
When an acid and base react with each other to form a salt and water, neutralisation reaction takes place. It is double displacement and highly exothermic in nature. Reaction between sulphuric acid and potassium hydroxide to form potassium sulphate and water is an example of neutralisation reaction:
\[\underset{(Sulphuric\,\,acid)}{\mathop{{{H}_{2}}S{{O}_{4}}}}\,+\underset{(potassium\,\,hydrodxide)}{\mathop{2KOH}}\,\xrightarrow{{}}\underset{(potassium\,\,sulphate)}{\mathop{{{K}_{2}}S{{O}_{4}}}}\,+\underset{(Water)}{\mathop{2{{H}_{2}}O}}\,\]
Oxidation-Reduction Reaction
Oxidation is defined as the addition of oxygen to an element or compound. It can also be defined as the removal of hydrogen from a compound.
Reduction is defined as the addition of hydrogen to an element or compound. It can also be defined as the removal of oxygen from a compound.
For example: The reaction between hydrogen sulphide and chlorine is an oxidation- reduction reaction.
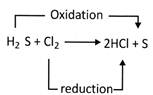
In the above reaction \[{{H}_{2}}S\] is oxidized to S because this process involves the removal of hydrogen from\[{{H}_{2}}S\]. On the other hand\[Cl\], is reduced to \[HCl\]because the process involves the addition of hydrogen to it. Hence, in this reaction \[{{H}_{2}}S\] is the reducing agent, whereas\[Cl\], is the oxidizing agent.
Oxidation in Daily Life
Corrosion: is a natural process, in which loss of electrons of metals takes places in the presence of water and oxygen. For example, the formation of a green coating on copper and brass, the tarnishing of silver when exposed to hydrogen sulphide gas, etc.
Rusting of Iron
Iron is very prone to rusting. Rusting of iron consists of the formation of hydrated iron oxide, \[Fe{{(OH)}_{3}}\]or\[F{{e}_{2}}{{O}_{3}}.{{H}_{2}}O.\]Rusting is a process that takes place in the presence of water. If any one of these is absent then rusting can't take place oxygen.
\[4Fe(s)+3{{O}_{2}}(g)+x.{{H}_{2}}O\to F{{e}_{2}}{{O}_{3}}.x{{H}_{2}}O\]
Acids
In everyday life, we use many substances that contain acids. For example, citrus fruits contain citric acid. Vinegar contains acetic acid.
In any chemistry laboratory, some common acids such as hydrochloric acid, sulphuric acid and nitric acid are used. These acids are called mineral acids because they can be prepared from naturally occurring compounds called minerals. The term acid, is derived from the Latin term acere, which means "sour". In the seventeenth century, the Irish writer and amateur chemist Robert Boyle first labelled substances as acids and bases.
Acids Can be Classified as Follows
All sour things that we use in our daily food contain acids. These acids are organic acids. Some of the common acids that are generally used in the laboratory are hydrochloric acid\[(HCl)\], sulphuric acid \[({{H}_{2}}S{{O}_{4}})\] and nitric acid\[(HN{{O}_{3}})\]. These are inorganic acids, also called mineral acids. Hydrochloric acid is also present in the gastric juice in our stomach.
Concentrated and Dilute Acids
An acid solution may be concentrated or dilute depending upon the amount of the acid present in the solution.
An acid is generally used as solution in water. When the solution contains a larger amount of the acid, it is said to be concentrated, whereas a dilute solution contains smaller amount of the acid.
Strong and Weak Acids
Some of the acids, when dissolved in water, get almost completely dissociated to provide hydrogen ions. These acids are called strong acids. For example, hydrochloric acid\[(HCl)\], nitric acid \[(HN{{O}_{3}})\] and sulphuric acid\[({{H}_{2}}S{{O}_{4}})\] are strong acids.
On the other hand, there are some acids which when dissolved in water are incompletely dissociated to give hydrogen ions. These are called weak acids. For example, carbonic acid \[({{H}_{2}}C{{O}_{3}})\] and acetic acid \[(C{{H}_{3}}COOH)\] are weak acids.
Acids have the Following Characteristics:
\[HCl={{H}^{+}}+C{{l}^{-}}\]
\[C{{H}_{3}}COOH={{H}^{+}}+C{{H}_{3}}CO{{O}^{-}}\]
Bases
Bases are ionic compounds that break apart to form a negatively charged hydroxide ion \[(O{{H}^{-}})\] in water. The strength of a base is determined by the concentration of hydroxide ions\[(O{{H}^{-}})\]. The greater the concentration of \[O{{H}^{-}}\]ions, the stronger the base.
A base is a substance, usually the oxide or the hydroxide of a metal, which can react with an acid to produce salt and water.
Bases have the following characteristics:
Examples of bases are sodium hydroxide, soap, slaked lime, etc.
In the late 1800s, the Swedish scientist Svante Arrhenius proposed that water can dissolve many compounds by separating them into their individual ions.
For example, hydrochloric acid \[(HCl)\] dissolves in water, as follows:
\[HCl\to {{H}^{+}}(aq)+C{{l}^{-}}(aq)\]
Arrhenius defined bases as substances that dissolve in water to release hydroxide ions \[(O{{H}^{-}})\] into solution. For example, a typical base according to the Arrhenius definition is sodium hydroxide\[(NaOH)\]:
\[NaOH\,\to \,N{{a}^{+}}(aq)\,+\,O{{H}^{-}}(aq)\]
The Arrhenius definition of acids and bases explains a number of things. Arrhenius's theory explains the reason for all acids having similar properties to each other and, conversely, why all bases are similar? This reason for all acids having similar properties are that all acids release \[{{H}^{+}}\] ions into solution. Similarly, because all bases release \[O{{H}^{-}}\]ions therefore have properties similar to each other. The reaction between acid and base is called neutralisation.
Uses of Bases
|
Bases |
Uses |
|
Sodium hydroxide |
1. In the manufacturing of soaps, textiles, paper, medicines 2. In the refining of petroleum |
|
Ammonium hydroxide |
1. As a reagent in the laboratory 2. In making fertilizers, rayon, plastics and dyes |
|
Calcium hydroxide |
1. In making cement and mortar 2. In making bleaching powder 3. In whitewashing 4. In removing acidity of soils |
Salt
A salt is a compound formed by the reaction of an acid with a base in which the hydrogen of the acid is replaced by the metal.
There are chemical compounds that are classified as salts. Sodium chloride or table salt is the most common example of salt. Baking soda is the salt of sodium bicarbonate. Magnesium sulphate, also called epsom salts is often found in the home.
Salts are ionic compounds that are composed of metallic ions and nonmetallic ions. For example, sodium chloride is composed of metallic sodium ions and nonmetallic chloride ions. Some salts are composed of metallic polyatomic ions and nonmetallic polyatomic ions. For example, ammonium nitrate is composed of metallic ammonium ions and non-metallic nitrate ions.
Properties of Salts
The salty taste of ocean water is due to the presence of salts such as sodium chloride and magnesium bromide. There are many different salts present in sea water.
Salts dissociate in water. Salts consist of tightly bonded ions. In water, these ionic bonds are weakened and therefore the ions become mobile. This weakening of ionic bond in water is the reason why salt solutions are electrolytes. For example, following reaction explains how sodium chloride ionises or dissociates in water.
\[NaCl(s)\to N{{a}^{+}}(aq)+C{{l}^{-}}(aq)\]
When sodium carbonate dissolves in water, the salt liberates sodium ions and carbonate ions. At the same time, the water itself ionises slightly to form hydrogen and hydroxide ions (remember that water is a weak electrolyte).
\[N{{a}_{2}}C{{O}_{3}}(s)\to 2N{{a}^{+}}(aq)+CO_{3}^{2-}(aq)\]
\[{{H}_{2}}O\,\to \,{{H}^{+}}\,(aq)\,+\,O{{H}^{-}}(aq)\]
Neutralisation of Acid and Base
Reaction between acid and base is a neutralization reaction, in which the acid and base neutralize each other. A neutralisation reaction, results in the formation of water and salt. For example, when sodium hydroxide and hydrochloric acid react with water, the common salt (sodium chloride) is formed.
Direct Combination
Reaction between a metal and a nonmetal, produces salt. For example, when magnesium is burned in chlorine gas, the salt magnesium chloride is formed.
Metal Oxide and Acid
Reaction between a metal oxide and acid produces a salt. For example, when calcium oxide reacts with nitric acid, the salt calcium nitrate is formed.
Uses of Salts
The following table lists uses of some salts:
|
Salts |
Uses |
|
Sodium bicarbonate |
1. An essential requirement of our food |
|
Sodium carbonate |
2. In the preservation of food |
|
Sodium bicarbonate |
3. In making a freezing mixture which is used by ice-cream vendors |
|
Potassium nitrate |
1. To make gunpowder, fireworks and glasses 2. As a fertilizer in agriculture |
|
Copper sulphate |
1. Commonly called ?blue vitriol?, used as a fungicide to kill certain germs 2. In electroplating 3. In dyeing |
|
Potash alum |
1. Used to purify water, makes suspended particles in water to settle down 2. As an antiseptic 3. In dyeing |
pH
According to the Bronsted-Lowry definition, the concentration of hydrogen ions present relates both acids and bases with each other. Acids increase the concentration of hydrogen ions, while bases decrease the concentration of hydrogen ions by accepting them. Therefore, the concentration of hydrogen ion can be the criteria for the acidity or basicity of a substance. In 1909, the Danish biochemist Soren Sorensen invented the pH scale for measuring acidity. The pH scale is described by the formula: log
\[pH=-\log \,\,[{{H}^{+}}]\]
Concentration is commonly abbreviated by using square brackets, thus\[[{{H}^{+}}]\] = hydrogen ion concentration.
For example, a solution with \[[{{H}^{+}}]=1\times {{10}^{-7}}\]moles/liter has a pH equal to 7 (a simpler way to think about pH is that it equals the exponent on the \[{{H}^{+}}\] concentration, ignoring the minus sign).
The pH scale ranges from 0 to 14. Substances with a pH between 0 and less than 7 are acids. Substances with a pH greater than 7 and up to 14 are bases. Substances with pH = 7, are neutral substances, for example, pure water.
You need to login to perform this action.
You will be redirected in
3 sec
