Thermodynamics and Heat Transfer
Category : Railways
Thermodynamics and Heat Transfer
THERMO DYNAMICS
In the subject of thermodynamics, the inter-relationship among heat, work and system properties are studied. It is also called as the conceptual science of entropy and energy.
Some Thermodynamical Terms in brief
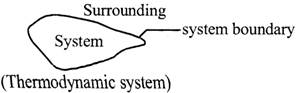
(i) Thermodynamic system: A thermodynamical system is an assembly of large number of particles which can be described by thermodynamic variables like pressure (P), volume(V), temperature(7).
(ii) Surroundings: Everything outside the system which can have a direct effect on the system is called surroundings. The gas cylinder in the kitchen is the thermodynamic system and the relevant part of the kitchen is the surroundings.
(iii) An adiabatic wall: The wall which prevent the passage of matter and energy.
(iv) Diathermic wall: It prevent the passage of matter but allow the passage of energy. An aluminium can is an example of a container whose walls are diathermic.
(v) Closed and open system: In a closed system, energy may transfer the boundaries of system but mass does not cross the boundary, while in open system, both mass and energy transfer across the boundary of the system.
(vi) An isolated system: hi this type of system neither the mass nor the energy can be exchanged with the surroundings.
(vii) Equation of state: The relationship between the pressure, volume and temperature of the thermodynamical system is called equation of state.
(viii) Properties: A property of a system is any abusable characteristic of the given system various properties of the system depend on the state of the system not on how that state have been reached.
(xi) Intensive property of a system or those properties whose values does not depend upon the mass of the system. Eg: Pressure, temperature, viscosity etc., while extensive properties depend upon the mass of the system. Eg: Length, volume etc.
(x) Equilibrium: A system is said to be in thermodynamic equilibrium when it does not lead to change its properties (macroscopic) and make balance with its surroundings. There, a system in mechanical, thermal and chemical equilibrium is said to be in thermodynamic equilibrium.
THERMODYNAMIC SYSTEM
A thermodynamic system is described as a kind of a region available in space and this region is concentrated for the purpose of analysing a problem. The system is considered to be separated from surroundings (external to system) by the boundary of the system. The nature of the boundary may be real or imaginary and it is considered to be flexible
i.e., it can change its shape or size. If we combine a system and its surroundings, then it constitutes the universe.
TYPES OF THERMODYNAMIC SYSTEMS:
There are three types of thermodynamic systems:
(a) Closed system:
A thermodynamic system in which mass is not transferred across system boundary but energy may be transferred in and out of the system, is known as closed system. Mass in the piston - cylinder arrangement is the example of a closed system.
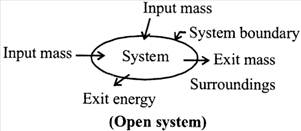
(b) Open system:
The open system is defined as a system in which mass as well as energy can be transferred with its surroundings. Open systems are most common. The region where analysis of the system is performed is known to be a control volume and the boundary of control volume is known as control surface. Eg: Air compressor
(c) Isolated system:
In an isolated system, no mass and no energy is transferred across system boundary.

(No mass transfer
No energy transfer)
(Isolated system)
ZEROTH LAW OF THERMODYNAMICS
If objects A and B are separately in thermal equilibrium with a third object C then objects A and B are in thermal equilibrium with each other.
Zeroth law of thermodynamics introduces thermodynamic quantity called temperature. Two objects (or systems) are said to be in thermal equilibrium if their temperatures are the same. In measuring the temperature of a body, it is important that the thermometer be in the thermal equilibrium with the body whose temperature is to be measured.
FIRST LAW OF THERMODYNAMICS
The first law of thermodynamics is based on conservation of energy. According to this law heat Q supplied to a system is equal to the sum of the change in internal energy (U) and work done by the system (W). Thus we can write
More about First Law of Thermodynamics
dQ = dU+dW
or dQ = dU+PdV.
SECOND LAW OF THERMODYNAMICS
(i) Kelvin – Plank Statement; It is impossible to construct an engine that can convert heat completely into work without producing any other effect. According to the statement the efficiency of any heat engine always be less than 100%.
(ii) Clausius Statement: For a self acting, machine, it is impossible to transfer heat from a colder body to a hotter body without the aid of external agency.
ENTROPY
Entropy is the another thermodynamical variable which many times very useful to understand the system. Entropy is related to the disorder or randomness in the system. To understand this, let us consider two systems as shown in Fig.
System 1 System 2
Ifandarc the entropies of the system 1 and 2 respectively at any temperature, then
(i) Entropy is not a conserved quantity.
(ii) Entropy can be created but cannot be destroyed.
(iii) Entropy of the universe always increases.
If a system at temperature Tis supplied a small amount of heat, then change in entropy of the system can be defined as ![]() for constant T
for constant T
For a system with variable T, we have
![]()
The second law of thermodynamics may be stated in terms of entropy as:
It is impossible to have a process in which the entropy of cm isolated system is decreased.
THERMODYNAMICAL PROCESSES
Any process may have own equation of state, but each thermodynamical process must obey PV= nRT.
Isabaric Process:
If a thermodynamic system undergoes physical change ai constant pressure, then the process is called isobaric.
Isochoric or Isometric Process:
A thermodynamical process in which volume of the system remain constant, is called isochoric process.
Isothermal Process:
A thermodynamical process in which pressure and volume of the system change at constant temperature, is called isothermal process.
Adiabatic Process:
An adiabatic process is one in which pressure, volume and temperature of the system change but heat will not exchange, between system and surroundings.
P–V Diagram Representing Four Different Processes
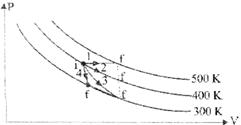
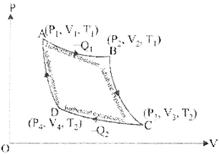
CARNOT CYCLE
Garnot cycle also has four above process. Thermodynamic coordinates after each operation are shown in Fig. Initially at A coordinates are ![]()
Enthalpy: Enthalpy is regarded as the total energy oil thermodynamic system. It is defined as the sum of interne energy and product of pressure and volume. It is an intensive property of the system. It is describe as:
H=U+PV
or
h = u + pv
where, H = Total enthalpy
U = Total internal energy
P = Pressure, V = Volume (Total)
h = specific enthalpy
u = specific internal energy
v= specific volume
Enthalpy is also considered as a function of temperature for the case of perfect gases. Hence, it can be written as:
![]()
where, ![]() enthalpy difference
enthalpy difference
![]() temperature difference
temperature difference
![]() specific heat at constant pressure
specific heat at constant pressure
REVERSIBLE AND IRREVERSIBLE PROCESSES
Reversible Process
Any process which can be made to proceed in the reverse direction by variation in its conditions such that any change occurring in any part of the direct process is exactly reversed in the corresponding part of reverse process is called a reversible process.
Examples:
(i) An infimitesimally slow compression and expansion of an ideal gas at constant temperature.
(ii) The process of gradual compression and extension of an elastic spring is approximately reversible.
(iii) A working substance taken along the complete Carnot's cycle.
(iv) The process of electrolysis is reversible if the resistance offered by the electrolyte is negligibly small.
A complete reversible process is an idealised concept as it can never be realised because dissipative forces cannot be completely eliminated.
Irreversible Process
Any process which cannot be retraced in the reverse direction exactly is called an irreversible process. Most of the processes occurring in the nature are irreversible processes.
Examples:
(i) Diffusion of gases.
(ii) Dissolution of salt in water.
(iii) Rusting of iron.
(iv) Sudden expansion or contraction of a gas.
BEHAVIOUR OF IDEAL AND REAL GASES
Behaviour of Ideal Gases
The behaviour of ideal gases is based on the following assumptions of kinetic theory of gases:
(1) All the molecules of a gas are identical. The molecules of different gases are different.
(2) The molecules are rigid and perfectly elastic spheres of very small diameter.
(3) Gas molecules occupy very small space. The actual volume occupied by the molecule is very small compared to the total volume of the gas. Therefore volume of the gas is equal to volume of the vessel.
(4) The molecules of gases are in a state of random motion, i.e., they are constantly moving with all possible velocities lying between zero and infinity in all possible directions.
(5) Normally no force acts between the molecules. Hence they move in straight line with constant speeds.
Equation of State or Ideal Gas Equation
The equation which relates the pressure (P), volume (V) and temperature (T) of the given state of an ideal gas is known as ideal gas equation or equation of state.
where R = universal gas constant
Numerical value of R= 8.31 joule mol"1 kelvur1
n=no. of moles of gas
Behaviour of Real Gases
The gases actually found in nature are called real gases,
![]()
![]()
Here a and b are Constant called Vander waal's constant.
PROPERTIES OF PURE SUBSTANCES
It is a single substance and has a uniform composition. It has constant chemical composition through its mass.
It has a same colour, taste and texture.
It has a fixed melting point and boiling point.
Types of Pure Substances
Two different types of pure substances are:
(i) Element: An element is a substance which cannot be split up into two or more simpler substances by usual chemical methods of applying heat, Sighting or electric energy, e.g., hydrogen, oxygen, sodium, chlorine etc.
(ii) Compound: A compound is a substance made up of two or more elements chemically combined in a fixed ratio by weight
e.g.![]() (water), Nad (sodium chloride) etc.
(water), Nad (sodium chloride) etc.
Energy conversion:
Energy transformation, also termed as energy conversion, is the process of changing energy from one of its forms into another. In physics, energy is a quantity that provides the capacity to perform many actions—some as simple as lifting or warming an object. In addition to being convertible, energy is transferable to a different location or object, but it cannot be created or destroyed.
Energy in many of its forms may be used in natural processes, or to provide some service to society such as heating, refrigeration, lightening or performing mechanical work to operate machines.
Heat transfer:
Heat transfer is a study of the exchange of thermal energy through a body or between bodies which occurs when there is a temperature difference. When two bodies are at different temperatures, thermal energy transfers from the one with higher temperature to the one with lower temperature. Heat always transfers from hot to cold.
Heat transfer affects the performance, emissions and durability of the engine as well as the design, packaging, material choice and fatigue life of vehicle components.
Table below shows the common SI and English units and conversion factors used for heat and heat transfer rates. Heat is typically given the symbol Q, and is expressed in joules (J) m SI units. The rate of heat transfer is measured in watts (W), equal to joules per second, and is denoted by q. The heat flux, or the rate of heat transfer per unit area, is measured in watts per area (W/ m2), and uses q" for the symbol.
Modes of Heat Transfer
There are three modes of heat transfer: conduction, convection, and radiation. Any energy exchange between bodies occurs through one of these modes or a combination of them.
Conduction is the transfer of heat through solids or stationery fluids. Convection uses the movement of fluids to transfer heat.
Radiation does not require a medium for transferring heat; this mode uses the electromagnetic radiation emitted by an object for exchanging heat.
Conduction
Conduction is at transfer through solids or stationery fluids. When you touch a hot object, the heat you feel is transferred through your skin by conduction. Two mechanisms explain how heat is transferred by conduction: lattice vibration and particle collision.
Conduction through solids occurs by a combination of the two mechanisms; heat is conducted through stationery fluids primarily by molecular collisions.
In solids, atoms are bound to each other by a series of bonds, analogous to springs as shown in Figure below.
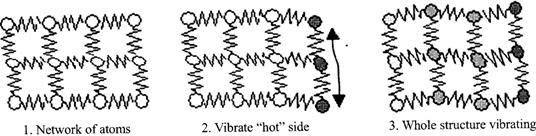
Convection
Convection uses the motion of fluids to transfer heat. In a typical convective heat transfer, a hot surface heats the surrounding fluid, which is then carried away by fluid movement such as wind.
The warm fluid is replaced by cooler fluid, which can draw more heat away from the surface. Since the heated fluid is constantly replaced by cooler fluid, the rate of heat transfer is enhanced. Natural convection (or free convection) refers to a case where the fluid movement is created by the warm fluid itself. The density of fluid decrease as it is heated; thus, hot fluids are lighter than cool fluids. Warm fluid surrounding a hot objects rises, and is replaced by cooler fluid. The result is a circulation of air above me warm surface.
Radiation
Radiative heat transfer does not require a medium to pass through; thus, it is the only form of heat transfer present in vacuum. It uses electromagnetic radiation (photons), which travels at the speed of light and is emitted by any matter with temperature above 0 degrees Kelvin (-273 °C). Radiative heat transfer occurs when the emitted radiation strikes another body and is absorbed. We all experience radiative heat transfer everyday; solar radiation, absorbed by our skin, is why we feel warmer in the sun than in the shade.
Heat Exchanger Principles in Automobiles
Most automotive heat exchangers are similar to shell and tube cross flow design, with multiple tube passes. But instead of having a defined shell around the tubes, with another controlled fluid forced across the tubes by means of a pump, there is no limited control volume for the shell. The tubes are open to the air and are dependent upon outside conditions.
Type of Automotive Heat Exchangers
Some types of Automotive Heat Exchangers include but are not limited to radiators, oil coolers and intercoolers. It is possible to use heat exchangers for almost any of the fluids in a vehicle. Air conditioners and heaters are also examples, however they are not restricted to vehicles.
A radiator is a cooling device used in the engine in which hot liquid flows through exposed pipes and transfers heat to the air by fans. Fins are used to conduct the heat from the tubes and transfer it to the air. The fluid used is typically a mixture of ethylene glycol, water and a small amount of corrosion reducer.
Oil coolers are used mainly in transmissions to keep the oil temperatures within safe limits.
Finally intercoolers are air-to-air or air-to-liquid heat exchangers.
They are used on turbocharged internal combustion engines to cool down the hot compressed air coming from the turbocharger.
Heat Conduction in Cylinders and Spheres:
Steady state heat transfer through pipes is in the normal direction to the wall surface (no significant heat transfer occurs in other directions). Therefore, the heat transfer can be modeled as steady state and one dimensional, and the temperature of the pipe will depend only on the radial direction, T = T (r).
Since, there is no heat generation in the layer and thermal conductivity is constant, the Fourier law becomes:
![]() (W)
(W)
![]()
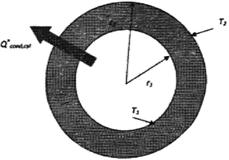
Fig.: Steady, one dimensional heat conduction in a cylindrical layer.
After integration:
![]()
![]()
![]()
![]()
Where![]() is the conduction resistance of the cylinder layer
is the conduction resistance of the cylinder layer
Following the analysis above, the conduction resistance for the spherical layer can be found:
![]()
The convection resistance remains the same in both cylindrical and spherical coordinates, ![]() However, note that the surface area
However, note that the surface area![]() (cylindrical) and
(cylindrical) and ![]() (spherical) are functions of radius.
(spherical) are functions of radius.
The Stefan-Boltzmann Law
The total power per unit area from a blackbody radiator can be obtained by integrating the Planck radiation formula over all wavelengths. The radiated power per unit area as a function of wavelength is
![]()
So the integrated power is
![]()
It is helpful to make the substitution
![]()
Making the substitution gives
![]()
Making use of the standard form integral
![]()
gives the final form of the Stefan-Boltzmann
![]()
So Stefan-Boltzmann Law:
![]()
Where E is the energy per time per area,
Constant of proportionality called the Stefan-Boltzmann constant.
Molecules constitute matter. Molecules made up of one or more atoms. The arrangement of atoms or molecules is different in solids, liquids and gases. The mean free path i.e., the average distance a molecule can travel without colliding is maximum in gases and least in solids. It is because the intermolecular distance in gases > liquids > solids.
KINETIC THEORY OF GASES
The theory is based on following assumptions as regards to the motion of molecules and the nature of the gases.
Assumptions of the kinetic theory of gases
(1) All the molecules of a gas are identical. The molecules of different gases are different.
(2) The molecules are rigid and perfectly elastic spheres of very small diameter.
(3) Gas molecules occupy very small space. The actual volume occupied by the molecule is very small compared to the total volume of the gas. Therefore volume of the gas is equal to volume of the vessel.
(4) The molecules of gases are in a state of random motion, i.e., they are constantly moving with all possible velocities lying between zero and infinity in all possible directions.
(5) Normally no force acts between the molecules. Hence they move in straight line with constant speeds.
(6) The molecules collide with one another and also with the walls of the container and change there direction and speed due to collision. These collisions are perfectly elastic i.e., there is no loss of kinetic energy in these collisions.
(7) The molecules do not exert any force of attraction or repulsion on each other except during collision. So, the molecules do not posses any potential energy. Their energy is wholly kinetic.
(8) The collisions are instantaneous i.e., the time spent by a molecule in a collision is very small as compared to the time elapsed between two consecutive collisions.
(9) Though the molecules are constantly moving from one place to another, the average number of molecules per unit volume of the gas remains constant.
(10) The molecules inside the vessel keep on moving continuously in all possible directions, the distribution of molecules in the whole vessel remains uniform.
(11) The mass of a molecule is negligibly small and the speed is very large, there is no effect of gravity on the motion of the molecules. If this effect were there, the density of the gas would have been greater at the bottom of the vessel.
GAS LAWS FROM KINETIC THEORY
Boyle's law: According to this law. the product of the pressure and the volume of a given mass of gas at constant temperature is constant. PV = constant.
![]() or
or ![]()
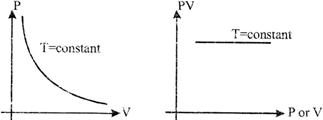
Boyle temperature: The temperature, at which the value of PV remains constant with respect to P, is defined as the Boyle temperature. The temperature at which the virial coefficient (?) becomes zero, is defined as the Boyle temperature.
Also the specific temperature at which the real gases obey Boyle's law, is defined as Boyle temperature.
i.e., Boyle temperature, ![]()
where a and b are constant
Critical temperature![]() : The limiting temperature at which a gas can be liquefied only by increasing the pressure and above which it cannot be liquefied, how large the pressure may be increased, is defined as critical temperature
: The limiting temperature at which a gas can be liquefied only by increasing the pressure and above which it cannot be liquefied, how large the pressure may be increased, is defined as critical temperature
Critical temperature, ![]()
Critical pressure![]() : The minimum pressure necessary to liquefied a gas at critical temperature is defined as critical pressure
: The minimum pressure necessary to liquefied a gas at critical temperature is defined as critical pressure
Critical pressure. ![]()
Critical volume![]() : The volume of 1 mol of a gas at critical pressure and critical temperature is defined as critical volume
: The volume of 1 mol of a gas at critical pressure and critical temperature is defined as critical volume
![]() . Critical volume,
. Critical volume, ![]()
Relation between ![]()
![]()
Values of a and b in terms of![]() ,
,![]() and
and![]()
![]()
Charle's law: According to this law, the volume of a given mass of gas at constant pressure is directly proportional to the absolute temperature of the gas.
If the pressure P is constant, then for a given mass of the gas, we have ![]() .
.
So,![]() or
or ![]()
Avogadro's law: Equal volumes of ?all' gases under the same conditions of temperature and pressure contain equal number of molecules.
![]()
Ideal Gas Equation
For![]() mol of any gas, PV =
mol of any gas, PV =![]() and
and
For 1gm.mol, PV=RT
![]()
![]()
For one gram of gas, ![]()
Value of R, its unit and dimensions:
![]()
Unit S.I.: joule/mol K.
Dimensions: ![]()
STEAM BOILERS
Steam boiler is basically a closed vessel into which water is heated until the water is converted into steam at required pressure by combustion of fuel. In this, fuel is generally burnt in a turnace and hot gases are produced. These hoi gases come in contact with water vessel and the heat of hot gases is transfered to water and steam is produced.
This steam is fed through pipes to the turbine of thermal power plant.
Applications
(i) Steam boilers are utilized as generators for the production of electricity in the energy sector.
(ii) Steam boilers are used in agriculture and soil - steaming.
(iii) Steam boilers are also used for heating the building in cold weather.
Classification of steam boilers
Steam boilers are classified based on the following basis:
(i) Steam pressure
(ii) Firing method
(iii) Tube contents
(iv) Circulation of water
(v) Heat source
(vi) Stationary or Portable
(vii) Position
(viii)Passage of gas
(ix) Draught nature
Difference between fire tube boiler and water tube boiler:
|
Fire tube boiler |
Water tube boiler |
|
Hot gases are inside the tubes while water outside the tubes |
Water is available inside the tube while hot gases outside the tubes. |
|
Firing is done internally |
Firing is done externally |
|
Operatinal pressure is upto 16 bar |
Operational pressure is high as upto 100 bar |
|
Less amout of steam is generated |
High amout of steam is generated |
|
It requires large floor area for given power |
It requires less floor area for the same power |
|
It is not generally used in large power plants |
It is used in large power plants |
|
Boiler shell diameter is large for given power |
Boiler shell diameter is small for the same amount of given power |
|
Parts are not accessible easily for the purpose of maintenance |
Parts are easily accessible for the purpose of maintenance |
|
Efficiency is less |
Efficiency is high |
|
Initial cost is less |
Initial cost is high |
|
It has a large ratio of water content to steam capacity |
It has a comparatively small ratio of water content to steam capacity |
|
Slow in evaporation |
Quick in eaporation |
COMPARISON BETWEEN IMPULSE AND REACTION TURBINE:
|
Impulse turbine |
Reaction turbine |
|
Only kinetic energy is utilized for the purpose of rotation of turbine |
Kinetic energy and pressure energy both are utilized for the purpose of rotation of turbine |
|
Water flows over the nozzie followed by striking followed by striking the blades |
Guide mechanism directs the water for the purpose of flowing over turbine |
|
Pressure is decreased in nozzle and not in moving blades |
Pressure is decreased in fixed blades and also in moving blades |
|
The type of the blades is profile |
The type of the blade is aerofiol |
|
Low power is |
High power is produced |
|
Low efficiency |
High efficiency |
|
For same power generations if requires less space |
For same power generations, it requires more space |
Internal combustion Engine(IC Engine):
An I.C. Engine is defined as a heat engine in which the combustion of fuel is occurred in the presence of air and this results in releasing the energy in the cylinder of an engine.
Comparison between I.C. Engine and Steam angine:
|
Steam Engine |
I.C. Engine |
|
In steam engine, combustion takes place outside the engine. |
In I.C. Engine, Combustion takes place inside the engine. |
|
Steam engines are operated at temperature values of around |
Operation of I.C. Engines done at temperature values of about |
|
It does not require cooling |
It requires cooling due to high operational temperatures |
|
The enhaust of steam engine is utilized as tgeed water |
The exhaust of an I.C. Engine is exited to the atmosphere. |
|
Instantaneous use for steam engine is not possible |
Instantaneous use of I.C.Engine is possible |
|
Weight to power ratio is high |
Weight to power ratio is low |
|
Steam engine has a very low value of efficiency i.e. 15-20% |
I.C. Engine has higher value of efficiency i.e. 30-36% |
Difference between four stroke and two storke cycle:
|
Four stroke cycle |
Two stroke cycle |
|
Cycle is completive in four strokes |
Cycle is combusted in two strokes |
|
Heavy flywheel is required |
Light flywheel is required |
|
Engine is heavy for same power output |
Engine is light for same power output |
|
Less cooling and lubrication is needed |
High cooling and lubrication is needed |
|
High initial cost |
Low initial cost |
|
High thermal efficiency |
Lower thermal efficiency |
|
Volumetric efficiency is more |
Volumetric efficiency is less |
Difference between SI and CI Engine:
|
S.I Engine |
C.I. Engine |
|
If performs on OHO cycle |
If performs on diesel cycle |
|
Compression ratio ranges from 5 to 10 |
Compression ratio ranges from 13 to 27 |
|
Carburetor supplies fuel |
Fuel injector supplies fuel |
|
Maintenance cost is low but running cost is high |
Maintenance cost is high but running cost is low |
|
Spark plug is used |
No spark plug is used |
|
Thermal efficiency is low |
Thermal efficiency is high |
Engine lubrication:
Lubrication is defined as a method in which oil is provided between two moving surfaces having relative motion between them. lubrication is employed to reduce friction between moving parts, There is an oil film made which acts like a cushion for moving parts and absorbs heat from the parts..
The basic characteristic of lubricant is viscosity, oiliness. Chemical stability, adhesiveness, film strength, flash point first point etc.
Kinds of lubricants
(i) Oils Suchas:
Mineral oils, fatty oils, synthetic, multigrade oils
(ii) Greases
![]() Lubricating grease
Lubricating grease
Eg. Aluminium, calcium, sodium greases
Engine Cooling
Engine cooling is very necessary to maintain the temperature of the engine low other it affects the behaviour of fuel combustion and also shortens the life of engine
Cooling system: there are generally two types of cooling systems used as:
(i) Air cooling
(ii) Water or liquid cooling
(i) Air cooling: In air cooling system. Air flows through the outsides of cylinder barrel and out surface area is increased by the use of times. The heat dissipated amout to air depends upon amout of air flowing through cooling tins, tin surface area and thermal conductivity of material of the fins used.
Applications: small engines, industrial and agricultural engines.
(ii) water or liquid cooling: In this system, water or liquid is made to circulate around the cylinders and thus absorbing heat from the walls of the cyclinder and cylinder head.coolant absorbs heat while passing thourgh the engine and lubricates the water pump.
![]() Methods for circulating water
Methods for circulating water
· Thermo syphon cooling, forced cooling, pressurized water cooling, evaporative cooling, thermostat cooling
![]() various components of water cooling system
various components of water cooling system
Applications: Industrial cooling towers. Marine vessel thyristors of HVDC value etc.
GOVERNING OF IC ENGINES
The process of providing any arrangement, which will keep the engine speed constant (according to the changing load conditions) is known as governing of I.C. engines. Though there are many methods for the governing of I.C. engines, yet the following are important:
Testing of 1C Engine:
In general, the purposes of testing an internal combustion engine are:
(i) To obtain information about the engine this cannot be determined by calculations.
(ii) To confirm data used in design, the validity of which is in doubt.
(iii) To satisfy the customer as to the rated power output with the guaranteed fuel consumption.
The majority of tests on internal combustion engines are carried out for commercial purposes in order to check the following:
(i) Rated power (brake power) with the guaranteed fuel consumption (kg/kW-hr.)
(u) The quantity of lubricating oil required on brake power basis per kW-hr.
(iii) The quantity of cooling water required on brake power basis in kg per kW-hr.
(iv) The steadiness of the engine when loaded at different loads.
(v) The overload carrying capacity of the engine.
REFGIGERATION AND AIR CONDITIONING
Refrigeration:
It can be defined as the process of transferring heat from a low temperature region to a high temperature region. In other words it is the process of cooling a substance. This can be achieved only if the heat is removed from that substance.
Refrigeration Cycle: Refrigeration is the process in which hear is removed from a body enclosed space so that its temperature is reduced and then maintained at the temperature below the surrounding temperature. The working substance which is used to produce refrigeration is known as refrigerant.
Heat Pump: Heat pump is used to remove heat from a body at lower temperature and transfer this heat to a body having high temperature on the expense of external work supplied.
Principle of refrigeration:
The principle of refrigeration is based on second law of thermodynamics. It sates that heat does not flow from a low temperature body to a high temperature body without the help of an external work.
In refrigeration process, since the heat has to be transferred from a low temperature body to a high temperature body some external work has to be done according to the second law of thermodynamics as shown. This external work is done by means of compressor, condenser etc.
The machie, which works under this principle an d serves the purpose of refrigeration is called a refrigerator.
Type of refrigerators:
Vapor compression refrigeration system:
Vapor Compression Refrigeration System: This type of refrigeration system is the most commonly used system in domestic refrigerators, m VCRS the vapor alternatively undergoes a change of phase from vapor to Construction:
Vapor compression refrigeration system has the following components at its basic parts.
Vapor Absorption Refrigeration System:
The compressor in the vapor compression refrigeration system consumes lot of energy. To avoid this, the vapor absorption refrigeration system has been developed. In this system, the compression process of vapor compression cycle is eliminated. Instead of that the three following process are introduced.
Applications of Refrigeration:
Domestic Refrigerators:
Most domestic refrigerators are of two types-either a single door fresh food refrigerator or a two-door refrigerator-freezer combination, with the freezer compartment on the top position of the cabinet, or a vertically split cabinet (side-by-side), with the freezer compartment on the left side of the cabinet. They are completely self-contained units and are easy to install. Most refrigerators useR-22 refrigerant, normally maintaining temperatures of 0°F in the freezer compartment and abouttoin the refrigerator compartment. The technician must be able to perform various duties in the maintenance and repair of domestic refrigerators, water coolers, and ice machines. This section provides information to aid you in handling some of the more common types of troubles. But let us remind you that the information given here is intended as a general guide and should, therefore, be used with the manufacturer's detailed instructions.
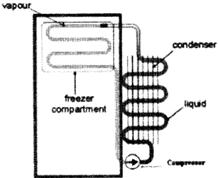
Single Door Fresh Food Refrigerator
A single door fresh food refrigerator consists of an evaporator placed either across the top or in one of the upper corners of the cabinet. The condenser is on the back of the cabinet or in the bottom of the cabinet below the hermetic compressor. During operation, the cold air from the evaporator flows by natural circulation through the refrigerated space. The shelves inside the cabinet are constructed so air can circulate freely past the ends and sides, elimination the need for a fan. This refrigerator has a manual defrost, which requires that the refrigerator be turned off periodically (usually overnight) to enable the buildup of frost on the evaporator to melt. Both the outside and inside finish is usually baked-on enamel. Porcelain enamel is found on steel cabinet liners. The interior of the unit contains the shelves, lights, thermostats, and temperature controls.
Two-Door Refrigerator-Freezer Combination
The two-door refrigerator-freezer combination is the most popular type of refrigerator. It is similar to the fresh food refrigerators in construction and the location of components except is sometimes has an evaporator for both the freezer compartment and be refrigerator compartment. Also, if it is a frost-free unit, the evaporators are on the outside of the cabinet.
Because of the two separate compartments (refrigerator-freezer) and the larger capacity, these types of refrigerators use forced air (fans) to circulate the air through the inside of both compartments. The tow-door refrigerator also has one of the following three types of evaporator defrost systems: manual defrost, automatic defrost, or frost-free. There are two types of automatic defrosting: the hot gas system of the electric heater system. The hot gas system, through the use of solenoid valves, uses the heat in the vapor from the compressor discharge line and the condenser to defrost the evaporator. The other system uses electric heaters to melt the ice on the evaporator surface. A frost-free refrigerator-freezer has the evaporator located outside the refrigerated compartment. On the running part of the cycle, air is drawn over the evaporator and is forced into the freezer and refrigerator compartments by a fan. On the off part of the cycle, the evaporators automatically defrost.
Refrigerator-freezer cabinets are made of pressed steel with a vinyl or plastic lining on the interior wall surfaces and a lacquer exterior finish. Most domestic refirgerators have urethane foam or fiber glass insulation in the cabinet walls. The side by side refrigerator- freezer arrangements has a number of features not found in other refrigerators. In addition to the automatic icemaker in the freezer compartment, it has an option for a cold water dispenser, a cube or crushed ice dispenser, and a liquid dispensed built into the door.
You need to login to perform this action.
You will be redirected in
3 sec
