A) \[{{\text{H}}_{2}}\text{, He}\]
B) \[{{\text{N}}_{\text{2}}}\text{, C}{{\text{H}}_{\text{4}}}\]
C) \[\text{He, C}{{\text{H}}_{4}}\]
D) \[{{\text{H}}_{2}}\text{, C}{{\text{H}}_{4}}\]
Correct Answer: C
Solution :
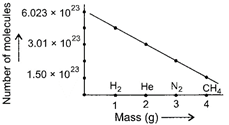
2 g of \[{{\text{H}}_{\text{2}}}\equiv 6.022\times {{10}^{23}}\] molecules 1 g of \[{{\text{H}}_{\text{2}}}\equiv \frac{6.022\times {{10}^{23}}}{2}\]molecules i.e., \[3.011\times {{10}^{23}}\] molecules \[4g\] of \[He\equiv 6.022\times {{10}^{23}}\]molecules \[2g\] of \[He\equiv \frac{6.022\times {{10}^{23}}}{4}\times 2\] molecules \[i.e.,\,\,3.011\times {{10}^{23}}\] molecules 28 g of \[{{N}_{2}}\equiv 6.022\times {{10}^{23}}\] molecules 3 g of \[{{N}_{2}}\equiv \frac{6.022\times {{10}^{23}}}{28}\times 3\] molecules i.e., \[0.6452\times {{10}^{23}}\] molecules 16 g of \[C{{H}_{4}}\equiv 6.022\times {{10}^{23}}\]molecules \[4g\,\,of\,\,C{{H}_{4}}\equiv \frac{6.022\times {{10}^{23}}}{16}\times 4\]molecules i.e., \[1.5055\times {{10}^{23}}\]molecules Thus, in graph, He and \[\text{C}{{\text{H}}_{\text{4}}}\]gases are placed at correct position.You need to login to perform this action.
You will be redirected in
3 sec
