A) octahedral, tetrahedral and square planar
B) tetrahedral, square planar and octahedral
C) square planar, tetrahedral and octahedral
D) octahedral, square planar and octahedral
Correct Answer: B
Solution :
[b] (i) \[N{{i}^{2+}}+4C{{l}^{-}}\to {{[Ni{{(Cl)}_{4}}]}^{2-}}\](Tetrahedral) \[Ni(Z=28)=3{{d}^{8}}4{{s}^{2}},N{{i}^{2+}}=3{{d}^{8}}\] Since \[C{{l}^{-}}\] ion is a weak ligand so pairing does not occur, thus \[s{{p}^{3}}\]hybridisation and is tetrahedral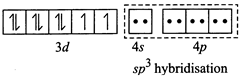 (ii) \[N{{i}^{2+}}+4C{{N}^{-}}\to {{[Ni{{(CN)}_{4}}]}^{2-}}\] (Square planar) Since \[C{{N}^{-}}\]ion is a weak ligand so pairing occurs, thus \[ds{{p}^{2}}\] hybridisation and is square planar
(ii) \[N{{i}^{2+}}+4C{{N}^{-}}\to {{[Ni{{(CN)}_{4}}]}^{2-}}\] (Square planar) Since \[C{{N}^{-}}\]ion is a weak ligand so pairing occurs, thus \[ds{{p}^{2}}\] hybridisation and is square planar 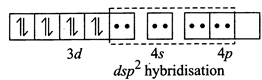 (iii) \[N{{i}^{2+}}+{{H}_{2}}O\to {{[Ni{{({{H}_{2}}O)}_{6}}]}^{2+}}\](octahedral) Since \[{{H}_{2}}O\] is a weak ligand so pairing does not occur, thus \[s{{p}^{3}}{{d}^{2}}\] (outer complex) hybridisation and is octahedral
(iii) \[N{{i}^{2+}}+{{H}_{2}}O\to {{[Ni{{({{H}_{2}}O)}_{6}}]}^{2+}}\](octahedral) Since \[{{H}_{2}}O\] is a weak ligand so pairing does not occur, thus \[s{{p}^{3}}{{d}^{2}}\] (outer complex) hybridisation and is octahedral 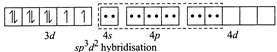
You need to login to perform this action.
You will be redirected in
3 sec
