A) pentagonal bipyramid
B) square pyramid
C) trigonal bipyramid
D) octahedral
Correct Answer: A
Solution :
There are 56 = ( = 7 + 7 x 7) valence electrons in 1F7. There with be 7 pairs of electrons around 1. These will be oriented in the directions of pentagonal bi pyramid as shown in the following structure.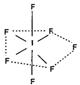
You need to login to perform this action.
You will be redirected in
3 sec
