A) \[2.73\times {{10}^{-2}}\,mol\,{{L}^{-1}}\]
B) \[3.74\times {{10}^{-\,3}}\,mol\,{{L}^{-1}}\]
C) \[4.75\times {{10}^{-\,3}}\,mol\,{{L}^{-1}}\]
D) \[5.27\times {{10}^{-\,3}}\,mol\,{{L}^{-1}}\]
Correct Answer: A
Solution :
| \[\Lambda _{m}^{c}=40.1\,\,\,S\,\,\,c{{m}^{2}}\,\,mo{{l}^{-1}}\] |
| \[\Lambda _{m}^{{}^\circ }=\Lambda _{m(HCO{{O}^{-}})}^{{}^\circ }+\Lambda _{m({{H}^{+}})}^{{}^\circ }\] |
| \[=(54.6+349.6)S\,c{{m}^{2}}mo{{l}^{-\,1}}\]\[=404.2\,S\,c{{m}^{2}}mo{{l}^{-\,1}}\] |
| \[\alpha =\frac{\Lambda _{m}^{c}}{\Lambda _{m}^{{}^\circ }}=\frac{40.1}{404.2}=0.0992\] |
| Now, |
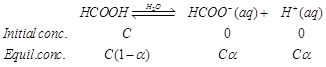 |
| \[{{K}_{\alpha }}=\frac{C{{\alpha }^{2}}}{1-\alpha }\] \[=\frac{0.25\times {{(0.0992)}^{2}}}{(1-(0.0992)}\]\[=2.73\times {{10}^{-\,3}}mol\,{{L}^{-\,1}}\] |
You need to login to perform this action.
You will be redirected in
3 sec
