A) Acidic strength of \[HBr>HCl\] but reverse is true for their reducing property
B) Basic strength of \[P{{H}_{3}}>As{{H}_{3}}\] but reverse is true for their Bond angle
C) Dipole moment of \[C{{H}_{3}}Cl>C{{H}_{3}}F\] but reverse is true for their HCH bond angle
D) \[{{K}_{{{a}_{1}}}}\]of fumaric acid is higher than maleic acid but reverse is true for their \[{{K}_{{{a}_{2}}}}\]
Correct Answer: C
Solution :
| [a] Acidic strength of HBr > HCl and their reducing properties are also in same order |
| [b] Basic strength of \[P{{H}_{3}}>As{{H}_{3}},\]their bond angles are also in same order \[{{\theta }_{2}}>{{\theta }_{1}}\] because \[C-H\] bond has more s-character in \[C{{H}_{3}}F\]than in \[C{{H}_{3}}Cl\] |
 |
| [D] \[{{K}_{{{a}_{1}}}}\]of maleic acid is higher then \[{{K}_{{{a}_{1}}}}\]of fumaric acid but reverse is true for their \[{{K}_{{{a}_{2}}}}\] |
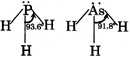 |
[C] 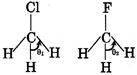 |
You need to login to perform this action.
You will be redirected in
3 sec
