A) \[BC{{l}_{3}}\]
B) \[CC{{l}_{4}}\]
C) \[PC{{l}_{5}}\]
D) \[BeC{{l}_{2}}\]
Correct Answer: A
Solution :
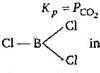 in \[BC{{l}_{3}}\] boron contains six electrons in its valence shell so, it is capable to accommodate one pair of electrons. Hence, it acts as Lewis acid or electron deficient compound. As we know that Lewis acids are the substances having a tendency to accept a pair of electron.
in \[BC{{l}_{3}}\] boron contains six electrons in its valence shell so, it is capable to accommodate one pair of electrons. Hence, it acts as Lewis acid or electron deficient compound. As we know that Lewis acids are the substances having a tendency to accept a pair of electron.
You need to login to perform this action.
You will be redirected in
3 sec
