A) \[NO_{2}^{-}\]
B) \[NO_{3}^{-}\]
C) \[PO_{4}^{3-}\]
D) \[CO_{3}^{2-}\]
Correct Answer: C
Solution :
In P?O bond, it bond is formed by the sidewise overlapping of d-orbital of P and p-orbital of oxygen. Hence, it is formed by \[p\pi -d\pi \] overlapping. In nitrogen and carbon no vacant d-orbital is present. So, they do not form \[p\pi -d\pi \] bond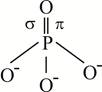
You need to login to perform this action.
You will be redirected in
3 sec
