A) \[{{\text{ }\!\![\!\!\text{ Ni(CN}{{\text{)}}_{\text{4}}}\text{ }\!\!]\!\!\text{ }}^{\text{2-}}}\]
B) \[{{\text{ }\!\![\!\!\text{ Cr(N}{{\text{H}}_{\text{3}}}{{\text{)}}_{6}}\text{ }\!\!]\!\!\text{ }}^{\text{3+}}}\]
C) \[{{\text{ }\!\![\!\!\text{ Fe(}{{\text{H}}_{2}}\text{O}{{\text{)}}_{6}}\text{ }\!\!]\!\!\text{ }}^{\text{2+}}}\]
D) \[{{\text{ }\!\![\!\!\text{ Ni(}{{\text{H}}_{2}}\text{O}{{\text{)}}_{6}}\text{ }\!\!]\!\!\text{ }}^{\text{2+}}}\]
Correct Answer: A
Solution :
Key Idea For the absorption of visible light, presence of unpaired d-electrons is the necessity. In \[{{[Ni{{(CN)}_{4}}]}^{2-}},Ni\] is present as \[N{{i}^{2+}}.\]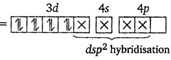 \[N{{i}^{2+}}=[Ar]\,3{{d}^{8}}4{{s}^{0}}\] \[\therefore {{[Ni{{(CN)}_{4}}]}^{2-}}\] (Pairing occurs because \[\text{C}{{\text{N}}^{-}}\]is a strong field ligand). Since, in \[{{[Ni{{(CN)}_{4}}]}^{2-}},\]no unpaired electron is present in d-orbitals, it does not absorb visible light. In\[{{[Cr{{(N{{H}_{3}})}_{6}}]}^{3+}}\]is present as \[C{{r}^{3+}}\] \[C{{r}^{3+}}=[Ar]3{{d}^{3}}4{{s}^{0}}\](Three unpaired electrons) In \[{{[Fe{{({{H}_{2}}O)}_{6}}]}^{2+}},\]Fe is present as \[F{{e}^{2+}}.\] \[F{{e}^{2+}}=[Ar]3{{d}^{6}}4{{s}^{0}}\] (Four unpaired electrons) In \[{{[Ni{{({{H}_{2}}O)}_{6}}]}^{2+}},Ni\]is present as \[N{{i}^{2+}}.\] \[N{{i}^{2+}}=[Ar]3{{d}^{8}}4{{s}^{o}}\](Two unpaired electrons) The complexes given in option (b), (c), (d) have unpaired electrons, thus absorb visible light.
\[N{{i}^{2+}}=[Ar]\,3{{d}^{8}}4{{s}^{0}}\] \[\therefore {{[Ni{{(CN)}_{4}}]}^{2-}}\] (Pairing occurs because \[\text{C}{{\text{N}}^{-}}\]is a strong field ligand). Since, in \[{{[Ni{{(CN)}_{4}}]}^{2-}},\]no unpaired electron is present in d-orbitals, it does not absorb visible light. In\[{{[Cr{{(N{{H}_{3}})}_{6}}]}^{3+}}\]is present as \[C{{r}^{3+}}\] \[C{{r}^{3+}}=[Ar]3{{d}^{3}}4{{s}^{0}}\](Three unpaired electrons) In \[{{[Fe{{({{H}_{2}}O)}_{6}}]}^{2+}},\]Fe is present as \[F{{e}^{2+}}.\] \[F{{e}^{2+}}=[Ar]3{{d}^{6}}4{{s}^{0}}\] (Four unpaired electrons) In \[{{[Ni{{({{H}_{2}}O)}_{6}}]}^{2+}},Ni\]is present as \[N{{i}^{2+}}.\] \[N{{i}^{2+}}=[Ar]3{{d}^{8}}4{{s}^{o}}\](Two unpaired electrons) The complexes given in option (b), (c), (d) have unpaired electrons, thus absorb visible light.
You need to login to perform this action.
You will be redirected in
3 sec
