question_answer 1) A car accelerates from rest at a constant rate \[\alpha \]for some time after which it decelerates at a constant rate \[\beta \] to come to rest. If the total time elapsed is t second, the maximum velocity reached is
A)
\[\frac{{{\alpha }^{2}}\beta t}{{{\alpha }^{2}}+{{\beta }^{2}}}\]
done
clear
B)
\[\frac{\alpha \beta t}{\alpha +\beta }\]
done
clear
C)
\[\frac{{{\beta }^{2}}\alpha }{{{\alpha }^{2}}+\beta }\]
done
clear
D)
\[\frac{\alpha \beta }{t}\]
done
clear
View Answer play_arrow
question_answer 2) The OR gate follows the Boolean expression, for A and B as two input variables and Y as output
A)
A + B = Y
done
clear
B)
A. B = V
done
clear
C)
\[\overline{A}\] = Y
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 3) If the frequency of the incident light is doubled, the kinetic energy of the emmitted electron will be
A)
zero
done
clear
B)
reduced to \[\frac{1}{3}\]
done
clear
C)
also doubled
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 4) If the two interfering beams have intensities in the ratio of 9 : 4. The ratio of intensities of maxima and minima in the interference pattern will be
A)
4 : 9
done
clear
B)
3 : 4
done
clear
C)
1 : 25
done
clear
D)
25 : 1
done
clear
View Answer play_arrow
question_answer 5) The charge of an electron is \[1.6\times {{10}^{-19}}C.\]How many electrons strike the screen of cathode ray tube each second, when the beam current is 16 m A?
A)
\[\text{1}{{0}^{-\text{19}}}\]
done
clear
B)
\[\text{1}{{0}^{-17}}\]
done
clear
C)
\[\text{1}{{0}^{-18}}\]
done
clear
D)
\[\text{1}{{0}^{17}}\]
done
clear
View Answer play_arrow
question_answer 6) A 500 kg horse pulls a cart of mass 1500 kg along a level road with an acceleration of \[\text{1 m}/{{\text{s}}^{\text{2}}}.\] If the coefficient of sliding friction 0.2. The horizontal force exerted by the earth on the horse is
A)
6000 N
done
clear
B)
5000 N
done
clear
C)
4000 N
done
clear
D)
3000 N
done
clear
View Answer play_arrow
question_answer 7) A object of mass 5 kg falls from rest through: a vertical distance of 20 m and reaches with a velocity of 10 m/s. How much work is done by push of air on the object? \[\left( \text{g }=\text{ 9}.\text{6 m}/{{\text{s}}^{\text{2}}} \right)\]
A)
-730 J
done
clear
B)
-365 J
done
clear
C)
-530 J
done
clear
D)
-1460 J
done
clear
View Answer play_arrow
question_answer 8) A 1.0 kg ball falls vertically on to the floor with a speed of 25 m/s. It rebounds with an initial speed of 10 m/s. Find the restitution coefficient and the impulse acting on the ball during contact, if the ball is in contact for 0.02 s. What is average exerted force on the floor ?
A)
0.1, 55 kg m/s, 1550 N
done
clear
B)
0.2, 45 kg m/s 1650 N
done
clear
C)
0.3, 30 kg m/s, 1550 N
done
clear
D)
0.4, 35 kg m/s, 1750 N
done
clear
View Answer play_arrow
question_answer 9) A substance reduces to 1/16th of its original mass in 2 h. The half-life period of the substance will be
A)
120 min
done
clear
B)
60 min
done
clear
C)
30 min
done
clear
D)
15 min
done
clear
View Answer play_arrow
question_answer 10) If the distance between the conduction band and valence band is 1 eV. This combination is
A)
semiconductor
done
clear
B)
conductor
done
clear
C)
metal
done
clear
D)
insulator
done
clear
View Answer play_arrow
question_answer 11) In a nuclear fission reaction
A)
A heavy nuclei breaks by itself
done
clear
B)
A heavy nuclei bombarded by thermal neutrons break up
done
clear
C)
A light nucleus bombarded by thermal neutrons break up
done
clear
D)
The light nucleus combine to produce a heavier nucleus
done
clear
View Answer play_arrow
question_answer 12) In 1865 the existance of electromagnetic waves was predicted by
A)
Maxwell
done
clear
B)
Hertz
done
clear
C)
Bose
done
clear
D)
Marconi
done
clear
View Answer play_arrow
question_answer 13) Two positive point charges of \[12\mu \,C\]and \[8\mu \,C\]are 10 cm apart. The work done in bringing them 4 cm closer, is
A)
1.3 eV
done
clear
B)
13 J
done
clear
C)
5.8 J
done
clear
D)
5.8 eV
done
clear
View Answer play_arrow
question_answer 14) Energy in Bohrs orbit is given by the \[{{E}_{n}}=-\left( \frac{B}{{{n}^{2}}} \right)\] where n is principal quantum number and \[\text{B}=\text{2}.\text{2}\times \text{1}{{0}^{\text{18}}}\text{ J}.\]The frequency of radiation when an electron jumps from the third orbit to the second orbit is \[\left( h=6.6\times \text{1}{{0}^{-34}}\text{ Js} \right)\]
A)
\[\text{4}.\text{6}\times \text{1}{{0}^{\text{14}}}\text{ Hz}\]
done
clear
B)
\[\text{2}.\text{3}\times \text{1}{{0}^{\text{14}}}\text{ Hz}\]
done
clear
C)
\[\text{8}.\text{2}\times \text{l}{{0}^{\text{14}}}\text{Hz}\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 15) The wavelength of the first member of the Balmer series of hydrogen spectrum is \[6563\,\overset{\text{o}}{\mathop{\text{A}}}\,\]. Calculate the wavelength of second member of the Paschen series in the same spectrum
A)
\[8563\,\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
B)
\[10818\,\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
C)
\[6409\,\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
D)
\[12818\,\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
View Answer play_arrow
question_answer 16) In a triode valve, the current in the plate circuit is controlled by
A)
ammeter
done
clear
B)
grid
done
clear
C)
cathode
done
clear
D)
anode
done
clear
View Answer play_arrow
question_answer 17) An electron beam passes through a magnetic field of \[\text{2}\times \text{1}{{0}^{-3}}\] \[\text{Wb}/{{\text{m}}^{\text{2}}}\]and an electric field of \[1.0\times \text{1}{{0}^{\text{4}}}\text{V}/\text{m}\]both acting simultaneously. The path of electron remains undeviated. The speed of electron, if the electric field is removed, and the radius of electron path will be respectively
A)
\[\text{1}0\times \text{1}{{0}^{\text{6}}}\text{ m}/\text{s},\text{ 2}.\text{43 cm}\]
done
clear
B)
\[\text{2}.\text{5}\times \text{1}{{0}^{\text{6}}}\text{ m}/\text{s},\text{ }0.\text{43 cm}\]
done
clear
C)
\[\text{5}\times \text{l}{{0}^{\text{6}}}\text{m}/\text{s},\text{ 1}.\text{42cm}\]
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 18) If the earth field induction at a place is 0.36 respectively gauss and angle of dip is \[60{}^\circ \]. The horizontal and vertical component of the field will be
A)
0.18 gauss, 0.18 \[\sqrt{3}\]gauss
done
clear
B)
0.09 gauss, 0.09 \[\sqrt{3}\]gauss
done
clear
C)
0.36 gauss, 0.36 \[\sqrt{3}\]gauss
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 19) A coil of mean area 500 \[c{{m}^{2}}\] and having 1000 turns is held perpendicular to a uniform field of 0.4 gauss. The coil is turned through \[180{}^\circ \] in s the average induced emf will be
A)
0.08 V
done
clear
B)
0.16 V
done
clear
C)
1.6 V
done
clear
D)
0.04 V
done
clear
View Answer play_arrow
question_answer 20) The internal resistance of a cell of emf 2 V is 0.10. It is connected to a resistance of 3.9 \[\Omega \] the voltage across the cell will be
A)
1.95 V
done
clear
B)
12.7 V
done
clear
C)
1.5 V
done
clear
D)
6.71 V
done
clear
View Answer play_arrow
question_answer 21) An inductance of 1 mH and a capacitance of 10 h F are connected in a circuit. The angular frequency of circuit will be
A)
\[\text{1}{{0}^{\text{3}}}\text{ rad}/\text{s}\]
done
clear
B)
\[\text{1}{{0}^{4}}\text{ rad}/\text{s}\]
done
clear
C)
\[\text{1}{{0}^{2}}\text{ rad}/\text{s}\]
done
clear
D)
\[\text{1}{{0}^{5}}\text{ rad}/\text{s}\]
done
clear
View Answer play_arrow
question_answer 22) If an AC main supply is given to be 220 V. The average emf during a positive half cycle will be
A)
198 V
done
clear
B)
220 V
done
clear
C)
240 V
done
clear
D)
220 \[\sqrt{2}\] V
done
clear
View Answer play_arrow
question_answer 23) A coil has an inductance of 0.7 H and is joined in series with a resistance of 220 f2. When the alternating emf of 220 V at 50 Hz is applied to it then the phase through which current lags behind the applied emf and the wattless component of current in the circuit will be respectively
A)
\[~30{}^\circ ,\text{ }1\text{ }A\]
done
clear
B)
\[~45{}^\circ ,\text{ }0.5\text{ }A\]
done
clear
C)
\[~69{}^\circ ,\text{ }1.5\text{ }A\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 24) A step up transformer operates on a 220 V line and supplies to a load of a current of 2 A. The ratio of turns in primary and the secondary winding is 25 : 1. The secondary voltage, primary current and power output will be respectively
A)
5500 V,50 A,11 kW
done
clear
B)
500 V, 25 A, 22 kW
done
clear
C)
2400 V, 40 A, 5.5 kW
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 25) Electron moves at right angle to a magnetic field of \[\text{1}.\text{5}\times \text{1}{{0}^{-\text{2}}}\] T with speed of \[\text{6}\times \text{1}{{0}^{\text{7}}}\text{ m}/\text{s}.\] If the specific charge of the electron is \[\text{1}.\text{7}\times \text{1}0\text{11 C}/\text{kg}.\] The radius of circular path will be
A)
3.31 cm
done
clear
B)
4.31 cm
done
clear
C)
1.31cm
done
clear
D)
2.35 cm
done
clear
View Answer play_arrow
question_answer 26) Find the number of photon emitted per second by a 25 W source of monochromatic light of wavelength \[6600\,\overset{\text{o}}{\mathop{\text{A}}}\,\]. What is the photoelectric current assuming 3% efficiency for photoelectric effect ?
A)
\[\frac{25}{3}\times {{10}^{19}}J,0.4\,A\]
done
clear
B)
\[\frac{25}{4}\times {{10}^{19}}J,6.2\,A\]
done
clear
C)
\[\frac{25}{2}\times {{10}^{19}}J,0.8\,A\]
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 27) The de-Broglie wavelength of thermal neutron is of the order of the
A)
size of grain
done
clear
B)
Bohrs radius
done
clear
C)
distance between atom in crystal
done
clear
D)
size of nucleus
done
clear
View Answer play_arrow
question_answer 28) Figure below shows regular hexagon, the charges are placed at the vertices. In which of the following cases the electric field at the centre is zero?
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 29) The equation of a wave moving on a string is \[\text{y}=8\text{ sin2}\,\pi \text{ }\left( 0.0\text{1 x}-\text{2 }\omega \text{t} \right),\] where y and x are in cm and t in second the amplitude of wave is
A)
8 cm
done
clear
B)
25 cm
done
clear
C)
50 cm
done
clear
D)
100 cm
done
clear
View Answer play_arrow
question_answer 30) The motion of particle is described by the equation\[x=a+b{{t}^{2}}\]where a = 15 cm and \[\text{b}=\text{3 cm}/\text{sb }=\text{ 3 cm}/{{\text{s}}^{\text{2}}}.\]Its instant velocityat time 3 s will be
A)
36 cm/s
done
clear
B)
9 cm/s
done
clear
C)
4.5 cm/s
done
clear
D)
18 cm/s
done
clear
View Answer play_arrow
question_answer 31) For a particle in a uniformly accelerated circular motion
A)
velocity is radial and acceleration has both radial and transverse components
done
clear
B)
velocity is transverse and acceleration has both radial and transverse components
done
clear
C)
velocity is radial and acceleration is transverse only
done
clear
D)
velocity is transverse and acceleration is radial only
done
clear
View Answer play_arrow
question_answer 32)
A long capillary tube of radius 1 mm open at both ends are filled with water and placed vertically. What will be the height of column of water left in capillary ?
A)
3 cm
done
clear
B)
1.5 cm
done
clear
C)
4.5 cm
done
clear
D)
6 cm
done
clear
View Answer play_arrow
question_answer 33) A steel wire of length 20 cm and uniform cross section 1 mm2 is tied rigidly at both the ends. If temperature of the wire is altered from \[40{}^\circ C\] to \[20{}^\circ C\]. Calculate the change in tension. (Given coefficient of linear expansion of steel \[\alpha =\text{1}.\text{1}\times {{10}^{-5}}/{}^\circ \text{C}\]and Youngs modulus Vfor steel \[=\text{ 2}\times \text{1}{{0}^{\text{11}}}\text{ N}/{{\text{m}}^{\text{2}}})\]
A)
88 N
done
clear
B)
UN
done
clear
C)
44 N
done
clear
D)
22 N
done
clear
View Answer play_arrow
question_answer 34) Two vessels of different materials are identical in size and wall thickness, they are filled with equal quantities of ice at \[0{}^\circ C\]. If the ice melts completely in 10 and 25 min respectively, then the coefficients of thermal conductivities of the material are
A)
2 : 3
done
clear
B)
3 : 5
done
clear
C)
5 : 2
done
clear
D)
1 : 1
done
clear
View Answer play_arrow
question_answer 35) If the root mean square velocity of the molecules of hydrogen at NTP is 1.84 km/s. Calculate the root mean square velocity of oxygen molecule at NTP molecule weight of hydrogen and oxygen are 2 and 32, respectively.
A)
1.47km/s .
done
clear
B)
0.94 km/s
done
clear
C)
1.84 km/s
done
clear
D)
0.47 km/s
done
clear
View Answer play_arrow
question_answer 36) The pressure p and density ratio of a diatomic gas \[\left( y=\frac{7}{5} \right)\]changes adiabatically from\[(\text{P},~\left( \text{p},\text{ }\rho \right).\text{ If }\frac{\rho }{\rho }=\text{32},\] then what is value of -\[\frac{p}{p}\] ?
A)
128
done
clear
B)
256
done
clear
C)
64
done
clear
D)
32
done
clear
View Answer play_arrow
question_answer 37) Calculate the work done when 1 mole of perfect gas is compressed adiabatically the initial pressure and volume of gas are \[\text{1}{{0}^{\text{5}}}\text{ N}/{{\text{m}}^{\text{2}}}\]and 6 L, respectively. The final value of the gas is 2 L molar specific heat of the gas at on constant volume is \[\frac{3R}{2}({{3}^{5/3}}=6.19)\]
A)
857 J
done
clear
B)
757 J
done
clear
C)
1057 J
done
clear
D)
957 J
done
clear
View Answer play_arrow
question_answer 38) Three bars of equal lengths and equal areas of cross-section are connected in series. Their thermal conductivities are in the ratio of 2 : 4 : 3. If the open ends of the first and the last bars are at temperatures \[200{}^\circ C\] and \[18{}^\circ C\] respectively in the steady state, the temperature of the two junctions is
A)
\[60{}^\circ C,\text{ }50{}^\circ C\]
done
clear
B)
\[116{}^\circ C,\text{ }74{}^\circ C\]
done
clear
C)
\[55{}^\circ C,\text{ }65{}^\circ C\]
done
clear
D)
\[160{}^\circ C,\text{ }80{}^\circ C\]
done
clear
View Answer play_arrow
question_answer 39) The temperature of a furnace is \[2324{}^\circ C\] and the intensity is maximum in its radiation spectrum nearly at \[12000\,\overset{\text{o}}{\mathop{\text{A}}}\,\]. If the intensity in the spectrum of a star is maximum nearly at \[4800\,\overset{\text{o}}{\mathop{\text{A}}}\,\]. Then surface temperature of the star is
A)
\[7219.5{}^\circ C\]
done
clear
B)
\[6219.5{}^\circ C\]
done
clear
C)
\[6319.5{}^\circ C\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 40) A thunder clap is heard 5.5s after the lightening flash. The distance of the flash is (velocity of sound in f...t = 330 m/s)
A)
1815m
done
clear
B)
1750m
done
clear
C)
4600m
done
clear
D)
3630m
done
clear
View Answer play_arrow
question_answer 41) A wave is expressed by the equation \[\text{y}=0.\text{5 sin it}\left( 0.0\text{1x}-\text{3t} \right),\]where x, y are in metres and t in seconds. The speed of propagation will be
A)
300 m/s
done
clear
B)
150 m/s
done
clear
C)
250 m/s
done
clear
D)
450 m/s
done
clear
View Answer play_arrow
question_answer 42) A stretched string of length 1 m and mass \[\text{5}\times \text{1}{{0}^{-4}}\text{ kg}\] fixed at both ends is under tension of 20 N. it is plucked at a point situated at 25 cm from one end. The frequency of vibration of the string will be
A)
125 Hz
done
clear
B)
50 Hz
done
clear
C)
200 Hz
done
clear
D)
100 Hz
done
clear
View Answer play_arrow
question_answer 43) A small air bubble is situated in a cube of 24 cm edge when viewed from one face, it appear to be 10 cm from the surface and through the opposite face is 6 m from the surface. Then the refractive index of the material of the cube is
A)
1.6
done
clear
B)
1.5
done
clear
C)
1.3
done
clear
D)
1.4
done
clear
View Answer play_arrow
question_answer 44) Light travels in two medium A and B with speeds \[\text{2}\times \text{l}{{0}^{\text{8}}}\text{ m}/\text{s}\]and \[\text{2}.\text{4}\times \text{l}{{0}^{\text{8}}}\text{ m}/\text{s}\] respectively. The critical angle C between them will be
A)
\[56.4{}^\circ \]
done
clear
B)
\[28.2{}^\circ \]
done
clear
C)
\[50.4{}^\circ \]
done
clear
D)
\[39.4{}^\circ \]
done
clear
View Answer play_arrow
question_answer 45) A thin prism Pi with angle \[6{}^\circ \] and made from glass of refractive index 1.54 is combined with another thin prism \[{{P}_{2}}\]of refractive index 1.72 to produce dispersion without deviation. The angle of prism \[{{P}_{2}}\]will be
A)
\[~4{}^\circ \text{ }30~'\]
done
clear
B)
\[8.5{}^\circ \]
done
clear
C)
\[6.5{}^\circ \]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 46) A person can see clearly objects lying between 15 cm and 100 cm from his eye. What will be the range of his vision, if he wear close fitting spectacles having a power of 0.8 D ?
A)
17 cm to 500 cm
done
clear
B)
34 cm to 250 cm
done
clear
C)
20 cm to 200 cm
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 47) A convergent lens of focal length 5 cm is used as a magnifying glass. If the image is formed at infinity. The magnifying power will be
A)
5
done
clear
B)
4
done
clear
C)
6
done
clear
D)
10
done
clear
View Answer play_arrow
question_answer 48) A ray of light is incident surface of a plate of glass of refractive index 1.5 at polarising angle. The angle of refraction of the ray will be
A)
\[60.2{}^\circ \]
done
clear
B)
\[16.8{}^\circ \]
done
clear
C)
\[43.7{}^\circ \]
done
clear
D)
\[33.7{}^\circ \]
done
clear
View Answer play_arrow
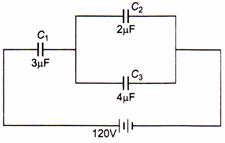
question_answer 49)
The charge on capacitors shown in figure and the potential difference across each will be respectively
A)
\[\text{24}0\mu \text{C},\text{8}0\mu \text{C},\text{16}0\mu \text{C and 8}0\text{ V},\text{ 4}0\text{ V},\text{ 4}0\text{V}\]
done
clear
B)
\[\text{3}00\mu \text{C},\text{75}\mu .\text{C},\text{15}0\mu \text{C and 4}0\text{ V},\text{ 8}0\text{ V},\text{ 6}0\text{V}\]
done
clear
C)
\[\text{22}0\mu \text{C},\text{7}0\mu \text{C},\text{14}0\mu \text{C and 6}0\text{ V},\text{ 5}0\text{ V},\text{ 4}0\text{V}\]
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 50) A current of 5A flows through an electric press of resistance 44 Cl. The energy consumed in 5 min is
A)
\[\text{3}.\text{3}\times \text{l}{{0}^{\text{5}}}\text{J}\]
done
clear
B)
\[2.5\times {{10}^{3}}J\]
done
clear
C)
\[1.5\times {{10}^{2}}J\]
done
clear
D)
\[{{10}^{8}}J\]
done
clear
View Answer play_arrow
question_answer 51) The correct value of the gas constant R is close to
A)
0.082 L-atm \[{{K}^{-1}}\,mo{{l}^{-1}}\]
done
clear
B)
0.082 \[L-at{{m}^{-1}}\] K mol
done
clear
C)
0.082 L-atm K mol
done
clear
D)
0.082 \[L-at{{m}^{-1}}\] K mol
done
clear
View Answer play_arrow
question_answer 52) Which one is correct for de-Broglie equation?
A)
\[E=\frac{\lambda }{p}\]
done
clear
B)
\[E=\frac{h}{\lambda }\]
done
clear
C)
\[E=\frac{h}{p}\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 53) It is possible to obtain oxygen from air by fractional distillation because
A)
oxygen has lower density than nitrogen
done
clear
B)
oxygen is in different group of periodic table from nitrogen
done
clear
C)
oxygen is more active than nitrogen
done
clear
D)
oxygen has higher boiling point than nitrogen
done
clear
View Answer play_arrow
question_answer 54) The rise in the boiling point of a solution containing 1.8 g of glucose in 100 g of a solvent is \[{{0.1}^{o}}C\]. The molal elevation constant of the liquid is
A)
0.01 K/m
done
clear
B)
0.1 K/m
done
clear
C)
IK/m
done
clear
D)
10 K/m
done
clear
View Answer play_arrow
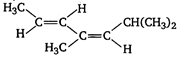
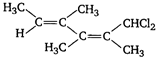
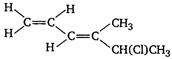
question_answer 55) Which one of the following will show optical activity?
A)
done
clear
B)
done
clear
C)
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 56) If the enthalpy of vaporisation of water is 186.5 J/mol, the entropy of its vaporization will be
A)
\[0.5\,J{{K}^{-1}}\,mo{{l}^{-1}}\]
done
clear
B)
\[1.0\,J{{K}^{-1}}\,mo{{l}^{-1}}\]
done
clear
C)
\[1.5\,J{{K}^{-1}}\,mo{{l}^{-1}}\]
done
clear
D)
\[2.0\,J{{K}^{-1}}\,mo{{l}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 57) Gibbs free energy G, enthalpy H, and entropy are related as
A)
\[G-TS=H\]
done
clear
B)
\[G=H+TS\]
done
clear
C)
\[S=H-G\]
done
clear
D)
\[G=H-TS\]
done
clear
View Answer play_arrow
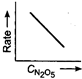
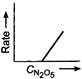
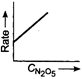
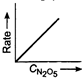
question_answer 58) Which one of the following plots is true for the first order decomposition of \[\,{{N}_{2}}{{O}_{5}}\]?
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 59) The half-life period for a zero order reaction is
A)
\[{{t}_{1/2}}\propto {{a}^{3}}\]
done
clear
B)
\[{{t}_{1/2}}\propto {{a}^{2}}\]
done
clear
C)
\[{{t}_{1/2}}\propto a\]
done
clear
D)
\[{{t}_{1/2}}\propto \frac{1}{a}\]
done
clear
View Answer play_arrow
question_answer 60) Which one of the following statements is correct for a positive catalyst?
A)
It initiates the reaction
done
clear
B)
It changes the state of equilibrium in favour of formation of product
done
clear
C)
It provides an alternative reaction path of lower activation energy
done
clear
D)
It provides activation energy to the reactants
done
clear
View Answer play_arrow
question_answer 61) The IUPAC name of the compound \[C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}-\underset{\begin{smallmatrix} || \\ O \end{smallmatrix}}{\mathop{C}}\,-C{{H}_{3}}\] based on latest nomenclature system is
A)
pentanone-2
done
clear
B)
pentan-2-one
done
clear
C)
2-pentanone
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 62) In the elimination reactions, the reactivity of alkyl halides follows the sequence
A)
\[R-F>R-I>R-Br>R-Cl\]
done
clear
B)
\[R-F>R-Cl>R-Br>R-I\]
done
clear
C)
\[R-I>R-Br>R-Cl>R-F\]
done
clear
D)
\[R-I>R-F>R-Br>R-Cl\]
done
clear
View Answer play_arrow
question_answer 63) The iodoform test is not given by
A)
\[C{{H}_{3}}-\underset{\begin{smallmatrix} | \\ O \end{smallmatrix}}{\mathop{C}}\,H-C{{H}_{3}}\]
done
clear
B)
\[C{{H}_{3}}-\overset{\begin{smallmatrix} O \\ || \end{smallmatrix}}{\mathop{C}}\,-C{{H}_{2}}-C{{H}_{3}}\]
done
clear
C)
\[C{{H}_{3}}C{{H}_{2}}OH\]
done
clear
D)
\[C{{H}_{3}}C{{H}_{2}}-\overset{\begin{smallmatrix} O \\ || \end{smallmatrix}}{\mathop{C}}\,-C{{H}_{2}}C{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 64) Sucrose on hydrolysis gives
A)
fructose + ribose
done
clear
B)
glucose + fructose
done
clear
C)
glucose + glucose
done
clear
D)
fructose + fructose
done
clear
View Answer play_arrow
question_answer 65) Which of the following has highest boiling point?
A)
\[{{(C{{H}_{3}})}_{3}}N\]
done
clear
B)
\[{{C}_{2}}{{H}_{5}}NHC{{H}_{3}}\]
done
clear
C)
\[C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}N{{H}_{2}}\]
done
clear
D)
\[N{{H}_{2}}C{{H}_{2}}C{{H}_{2}}N{{H}_{2}}\]
done
clear
View Answer play_arrow
question_answer 66) When alkyl halide reacts with aqueous sodium hydroxide to give alcohol, the reaction is called
A)
electrophilic substitution
done
clear
B)
nucleophilic substitution
done
clear
C)
free radical substitution
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 67) Which of the following does not undergo benzoin condensation?
A)
\[{{C}_{6}}{{H}_{5}}C{{H}_{2}}CHO\]
done
clear
B)
\[{{C}_{6}}{{H}_{5}}CHO\]
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 68) The reaction between an alkyl halide and an alkoxide ion to give an ether is known as
A)
Williamsons synthesis
done
clear
B)
Clemmensens reduction
done
clear
C)
Fittig reaction
done
clear
D)
Wurtz reaction
done
clear
View Answer play_arrow
question_answer 69) During bleaching of chlorine an antichlor is employed to
A)
eliminate last traces of bleaching agent
done
clear
B)
remove greases from the fibre
done
clear
C)
liberate oxygen
done
clear
D)
enhance bleaching action
done
clear
View Answer play_arrow
question_answer 70) The proper name for \[[Co{{(N{{H}_{3}})}_{6}}]C{{l}_{3}}\]
A)
hexamine cobalt trichloride
done
clear
B)
hexamine cobalt (III) chloride
done
clear
C)
hexamoniacobalt trichloride
done
clear
D)
cobalt hexamine chloride
done
clear
View Answer play_arrow
question_answer 71) Sequence of acidic character is
A)
\[{{N}_{2}}{{O}_{5}}>S{{O}_{2}}>CO>C{{O}_{2}}\]
done
clear
B)
\[{{N}_{2}}{{O}_{5}}>S{{O}_{2}}>C{{O}_{2}}>CO\]
done
clear
C)
\[S{{O}_{2}}>C{{O}_{2}}>CO>{{N}_{2}}{{O}_{5}}\]
done
clear
D)
\[S{{O}_{2}}>{{N}_{2}}{{O}_{5}}>CO>C{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 72) Which is a metalloid?
A)
Manganese
done
clear
B)
Phosphorus
done
clear
C)
Oxygen
done
clear
D)
Arsenic
done
clear
View Answer play_arrow
question_answer 73) The oxides, \[Cr{{O}_{3}},\,Mo{{O}_{3}}\] and \[W{{O}_{3}}\] are strongly
A)
neutral
done
clear
B)
acidic
done
clear
C)
basic
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 74) Which is the correct order of the first ionization potential of N, O and C?
A)
\[C>N>O\]
done
clear
B)
\[O>N>C\]
done
clear
C)
\[C<N>O\]
done
clear
D)
\[C>N\sim O\]
done
clear
View Answer play_arrow
question_answer 75) Among the following statements the incorrect one is
A)
zinc blende and iron pyrites are sulphides
done
clear
B)
calamine and siderite are carbonates
done
clear
C)
malachite and azurite are ores of copper
done
clear
D)
argentite and cuprite are oxides
done
clear
View Answer play_arrow
question_answer 76) The oxidation number of chlorine in \[HCl{{O}_{4}}\] is
A)
+7
done
clear
B)
+5
done
clear
C)
+3
done
clear
D)
-1
done
clear
View Answer play_arrow
question_answer 77) Nitrous acid reacts with \[{{H}_{2}}S{{O}_{4}}\] to give
A)
\[NO+S{{O}_{3}}\]
done
clear
B)
\[N{{O}_{2}}+S{{O}_{2}}\]
done
clear
C)
\[NO+S{{O}_{2}}\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 78) Pyrolusite is an ore of
A)
polonium
done
clear
B)
manganese
done
clear
C)
tin
done
clear
D)
lead
done
clear
View Answer play_arrow
question_answer 79) Turnbulls blue is a compound
A)
ferri cyanide
done
clear
B)
ferrous ferri cyanide
done
clear
C)
ferrous cyanide
done
clear
D)
ferri ferro cyanide
done
clear
View Answer play_arrow
question_answer 80) What is the composition of tear gas?
A)
\[KCl\,.\,\,MgC{{l}_{2}}24{{H}_{2}}O\]
done
clear
B)
\[N{{a}_{2}}{{S}_{2}}{{O}_{3}}\,.\,\,n{{H}_{2}}O\]
done
clear
C)
\[C{{l}_{3}}CN{{O}_{2}}\]
done
clear
D)
\[{{K}_{4}}Fe{{(CN)}_{6}}\]
done
clear
View Answer play_arrow
question_answer 81) Halogen having greatest electron affinity
A)
F
done
clear
B)
Cl
done
clear
C)
Br
done
clear
D)
I
done
clear
View Answer play_arrow
question_answer 82) Braggs law is given by the equation
A)
\[n\lambda =2d\sin \theta \]
done
clear
B)
\[\lambda =2\,nd\sin \theta \]
done
clear
C)
\[2n\,\lambda =d\sin \theta \]
done
clear
D)
\[n\lambda =2\theta \sin \theta \]
done
clear
View Answer play_arrow
question_answer 83) For a reaction to occur spontaneously
A)
\[\Delta T+T\Delta S\] must be negative
done
clear
B)
\[\Delta H-T\Delta S\] must be negative
done
clear
C)
\[\Delta S\] must be negative
done
clear
D)
\[\Delta H\] must be negative
done
clear
View Answer play_arrow
question_answer 84) Consider the equilibrium \[{{N}_{2}}(g)+3{{H}_{2}}(g)2N{{H}_{3}}(g);\,\Delta H=-93.6\,kJ\]The maximum yield of ammonia is obtained by
A)
increase of temperature and decrease of pressure
done
clear
B)
decrease of both the temperature and pressure
done
clear
C)
increase of both the temperature and pressure
done
clear
D)
decrease of temperature and increase of pressure
done
clear
View Answer play_arrow
question_answer 85) For the reaction,\[PC{{l}_{5}}(g)PC{{l}_{3}}(g)+C{{l}_{2}}(g)\]
A)
\[{{K}_{p}}={{K}_{c}}[RT]\]
done
clear
B)
\[{{K}_{p}}={{K}_{c}}{{[RT]}^{2}}\]
done
clear
C)
\[{{K}_{p}}={{K}_{c}}{{[RT]}^{-1}}\]
done
clear
D)
\[{{K}_{p}}={{K}_{c}}\]
done
clear
View Answer play_arrow
question_answer 86) A solid is formed from atom A and B and crystallizes into a face centred cubic lattice with A at the face centred position and B at the comers of the cube. Its molecular formula is represented by
A)
\[A{{B}_{3}}\]
done
clear
B)
\[{{A}_{3}}B\]
done
clear
C)
\[A{{B}_{2}}\]
done
clear
D)
\[{{A}_{2}}B\]
done
clear
View Answer play_arrow
question_answer 87) Which is not deflected by magnetic field?
A)
Proton
done
clear
B)
Neutron
done
clear
C)
Electron
done
clear
D)
Positron
done
clear
View Answer play_arrow
question_answer 88) Which of the following pairs are correctly matched?
A)
Isoelectronics \[{{N}^{3-}},{{O}^{2-}},C{{r}^{3-}}\]
done
clear
B)
Isotones \[_{14}S{{i}^{30}}{{,}_{15}}{{p}^{31}}{{,}_{16}}{{S}^{32}}\]
done
clear
C)
Isotopes \[_{20}C{{a}^{40}}{{,}_{19}}{{K}^{40}}\]
done
clear
D)
Isobars \[_{8}{{O}^{16}}{{,}_{8}}{{K}^{17}}{{,}_{8}}{{O}^{18}}\]
done
clear
View Answer play_arrow
question_answer 89) The weight of 11.2 L of \[C{{O}_{2}}\] at STP would be
A)
22 g
done
clear
B)
32 g
done
clear
C)
44 g
done
clear
D)
88 g
done
clear
View Answer play_arrow
question_answer 90) Addition of cone. \[HCl\] to saturated \[BaC{{l}_{2}}\]solution precipitates \[BaC{{l}_{2}}\]: because
A)
at constant temperature, the product \[[B{{a}^{2+}}]\,\,.\,C{{l}^{-2}}{{]}^{2}}\] remains constant in a saturated solution
done
clear
B)
ionic product \[[B{{a}^{2+}}]\,\,.\,\,[C{{l}^{-}}]\] remains constant in a saturated collection
done
clear
C)
the ionic product \[[B{{a}^{2+}}]\,{{[C{{l}^{-2}}]}^{2}}\] is greater than solubility product
done
clear
D)
it follows from Le-Chateliers principle
done
clear
View Answer play_arrow
question_answer 91) Which of the following will occur if a 0.1 M solution of a weak acid is diluted to 0.01 M at constant temperature?
A)
\[{{K}_{a}}\] will increase
done
clear
B)
pH will decrease
done
clear
C)
Percent ionisation increase
done
clear
D)
\[[{{H}^{+}}]\] will decrease to 0.01 M
done
clear
View Answer play_arrow
question_answer 92) In the equilibrium \[AB(s)A(g)+B(g)\] If the equilibrium concentration of A is doubled, the equilibrium concentration of B would become
A)
half
done
clear
B)
twice
done
clear
C)
\[\frac{1}{8}th\]
done
clear
D)
\[\frac{1}{4}th\]
done
clear
View Answer play_arrow
question_answer 93) The standard reduction potentials of some electrodes are \[{{E}^{o}}_{({{K}^{+}},K)}=-2.9\,V;\] \[{{E}^{o}}_{(Z{{n}^{2+}},Zn)}=-0.76\,\,V\] \[{{E}^{o}}_{\left( {{H}^{+}},\frac{1}{2}{{H}_{2}} \right)}=-0.0\] \[{{E}^{o}}_{(C{{u}^{2+}},Cu)}=-0.0\] The electrode acting as strong reductant is
A)
K
done
clear
B)
Cu
done
clear
C)
Zn
done
clear
D)
\[{{H}_{2}}\]
done
clear
View Answer play_arrow
question_answer 94) The freezing points of equimolar solutions of glucose, \[KN{{O}_{3}}\] and \[AlC{{l}_{3}}\] are in the order of
A)
\[AlC{{l}_{3}}<\] glucose \[<KN{{O}_{3}}\]
done
clear
B)
\[AlC{{l}_{3}}<KN{{O}_{3}}<\] glucose
done
clear
C)
glucose \[<KN{{O}_{3}}<AlC{{l}_{3}}\]
done
clear
D)
glucose \[<AlC{{l}_{3}}<KN{{O}_{3}}\]
done
clear
View Answer play_arrow
question_answer 95) What will be the molarity of a solution containing 5.0 g of \[NaOH\]in 250 mL solution?
A)
0.1
done
clear
B)
0.5
done
clear
C)
1.0
done
clear
D)
2.0
done
clear
View Answer play_arrow
question_answer 96) Which one of the following is the correct decreasing order of boiling point?
A)
\[{{H}_{2}}Te>{{H}_{2}}O>{{H}_{2}}Se>{{H}_{2}}S\]
done
clear
B)
\[{{H}_{2}}O>{{H}_{2}}S>{{H}_{2}}Se>{{H}_{2}}Te\]
done
clear
C)
\[{{H}_{2}}Te>{{H}_{2}}Se>{{H}_{2}}S>{{H}_{2}}O\]
done
clear
D)
\[{{H}_{2}}O>{{H}_{2}}Te>{{H}_{2}}Se>{{H}_{2}}S\]
done
clear
View Answer play_arrow
question_answer 97) van der Waals forces are applied to
A)
elementary gases only
done
clear
B)
mixture of gases
done
clear
C)
inert gases only
done
clear
D)
rare gases only
done
clear
View Answer play_arrow
question_answer 98) Among the following the most highly ionized in water is
A)
\[C{{H}_{2}}(Cl)C{{H}_{2}}C{{H}_{2}}COOH\]
done
clear
B)
\[C{{H}_{3}}C{{H}_{2}}CC{{l}_{2}}COOH\]
done
clear
C)
\[C{{H}_{3}}CH(Cl)C{{H}_{2}}COOH\]
done
clear
D)
\[C{{H}_{3}}C{{H}_{2}}CH(Cl)COOH\]
done
clear
View Answer play_arrow
question_answer 99) Sangars reagent is
A)
\[{{C}_{2}}{{H}_{5}}NH.N{{H}_{2}}\]
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 100) Geometrical isomerism is shown by
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 101) Bulk of RNA is synthesized in a cell during
A)
S - phase
done
clear
B)
\[{{G}_{1}}\]- phase
done
clear
C)
\[{{G}_{2}}\]- phase
done
clear
D)
M - phase
done
clear
View Answer play_arrow
question_answer 102) Other than DNA polymerase, which are the enzymes involved in DNA synthesis?
A)
Topoisomerase
done
clear
B)
Helicase
done
clear
C)
RNA primase
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 103) Which constitution is correct?
A)
RNA polymerase\[\to {{\alpha }_{2}}\beta {{\beta }^{1}}\delta \left( E.coli \right)\]
done
clear
B)
RNA polymerase\[\to {{\alpha }_{2}}\beta {{\beta }^{1}}\left( E.coli \right)\]
done
clear
C)
RNA polymerase\[\to {{\alpha }_{2}}\beta {{\beta }^{1}}\sigma \left( E.coli \right)\]
done
clear
D)
RNA polymerase\[\to {{\alpha }_{2}}{{\beta }^{2}}\sigma \left( E.coli \right)\]
done
clear
View Answer play_arrow
question_answer 104) The concept of "biological species" was proposed by
A)
Carolus Linnaeus
done
clear
B)
Darwin
done
clear
C)
E Mayer
done
clear
D)
Von Baer
done
clear
View Answer play_arrow
question_answer 105) In an experiment, an Amoeba was transferred from a container A to container B, Amoeba developed a contractile vacuole. However, the vacuole disappeared again when the Amoeba was transferred back to container A. The container A and B respectively contained
A)
fresh and marine water
done
clear
B)
marine and freshwater
done
clear
C)
Both contained freshwater
done
clear
D)
Both contained marine water
done
clear
View Answer play_arrow
question_answer 106) In normal atmosphere, the amount of oxygen in air and dissolved oxygen in water respectively are
A)
21% and 17%
done
clear
B)
95% and 15%
done
clear
C)
32% and 7%
done
clear
D)
67% and 13%
done
clear
View Answer play_arrow
question_answer 107) What is detected in the urine of a woman to confirm pregnancy?
A)
Progesterone
done
clear
B)
Insulins
done
clear
C)
HCG
done
clear
D)
Oxytocins
done
clear
View Answer play_arrow
question_answer 108) Which of these bile products is not used in the solubilization?
A)
Bile salts
done
clear
B)
Cholesterol
done
clear
C)
Lecithin
done
clear
D)
Bile pigments
done
clear
View Answer play_arrow
question_answer 109) Vitamin-A can be synthesized inside the body by
A)
stomach
done
clear
B)
pancreas
done
clear
C)
liver
done
clear
D)
spleen
done
clear
View Answer play_arrow
question_answer 110) One mg of thyroxin increases the metabolic rate to about
A)
2.5%
done
clear
B)
5%
done
clear
C)
10%
done
clear
D)
15%
done
clear
View Answer play_arrow
question_answer 111) Which of the following vitamins is used in electron transport system?
A)
Calciferol
done
clear
B)
Thiamine
done
clear
C)
Riboflavin
done
clear
D)
Ascorbic acid
done
clear
View Answer play_arrow
question_answer 112) Protein C, which inactivates factor VIII and thereby partly inhibits the clotting mechanism is activated by
A)
tissue factor pathway inhibitor
done
clear
B)
thrombin
done
clear
C)
antithrombin - III
done
clear
D)
heparin
done
clear
View Answer play_arrow
question_answer 113) The fusion of paternal and maternal sets of chromosomes of haploid gametes is known as
A)
amphimixis
done
clear
B)
coition
done
clear
C)
synkaryon
done
clear
D)
insemination
done
clear
View Answer play_arrow
question_answer 114) Which one of the following genotype has one barr body?
A)
XY
done
clear
B)
XX
done
clear
C)
XXX
done
clear
D)
XYY
done
clear
View Answer play_arrow
question_answer 115) In evolution, the resemblance between widely different or animals due to common adaptation may referred to as an example of
A)
convergent evolution
done
clear
B)
molecular analogy
done
clear
C)
molecular homology
done
clear
D)
homoplastic appearance
done
clear
View Answer play_arrow
question_answer 116) The interaction between a particular kind of organism and its environment is called
A)
ecosystem
done
clear
B)
niche
done
clear
C)
adaptation
done
clear
D)
biome groups
done
clear
View Answer play_arrow
question_answer 117) Excessive pulling of ligaments between two bones cause
A)
fracture of the bones
done
clear
B)
sprain
done
clear
C)
breakage of the muscles
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 118) Which of the vitamins cant be deposited Inside the body?
A)
Ascorbic acid
done
clear
B)
Calciferol
done
clear
C)
Vitamin-K
done
clear
D)
Retinol
done
clear
View Answer play_arrow
question_answer 119) Oenothera type of female gametophyte is always
A)
8 nucleate and 7 celled
done
clear
B)
16 nucleate and 13 celled
done
clear
C)
8 nucleate and 8 celled
done
clear
D)
4 nucleate and 4 celled
done
clear
View Answer play_arrow
question_answer 120) It is matter of common observation that monocots and dicots have one and two cotyledons respectively but in gymnosperms the number of cotyledons are
A)
always 3
done
clear
B)
3-8
done
clear
C)
2-5
done
clear
D)
variable 1-2
done
clear
View Answer play_arrow
question_answer 121) The neck of archegonium of Pinus roxburghii is made up of
A)
8 cells arranged in 4 tiers of 2 cells each
done
clear
B)
8 cells arranged in 2 tiers of 4 cells each
done
clear
C)
4 cells arranged in 2 tiers of 2 cells each
done
clear
D)
4 cells arranged in a single tier
done
clear
View Answer play_arrow
question_answer 122) If sporangia are developed from a single Initial cell, the development of sporangia is designated as
A)
eusporangiate
done
clear
B)
leptosporangiate
done
clear
C)
monosporangiate
done
clear
D)
polysporangiate
done
clear
View Answer play_arrow
question_answer 123) Turpentine oil, which is one of the most important raw material of paint and varnish industry is the product obtained from
A)
fractional distillation of petroleum
done
clear
B)
destructive distillation of wood
done
clear
C)
alpine forest
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 124) TLV (Threshold Limit Value) of methyl isocynate is
A)
0.002 ppm
done
clear
B)
0.02 ppm
done
clear
C)
0.2 ppm
done
clear
D)
0.005 ppm
done
clear
View Answer play_arrow
question_answer 125) Like angiospermic parasite such as Cuscuta, there are some parasitic forms of Rhodophyta, which are colourless, heterotrophic and grow on other members of Rhodophyta. Select which one is a parasitic form of red algae?
A)
Gelidium
done
clear
B)
Harveyella
done
clear
C)
Choridras
done
clear
D)
Both (a) and (b)
done
clear
View Answer play_arrow
question_answer 126) In the vast marine ecosystem, certain sea develops red coloration. This red color is due to the presence of large population of which one of the following organisms?
A)
Trichodesmium erythrium
done
clear
B)
Physarium
done
clear
C)
Dinoflagellates
done
clear
D)
Diatoms and members of red algae
done
clear
View Answer play_arrow
question_answer 127) Megasporophylls, which are arranged spirally and acropetally on the central axis of the female cone of Pinus bear
A)
4 megasporangia on dorsal surface
done
clear
B)
2 megasporangia on ventral surface
done
clear
C)
2 megasporangia on dorsal surface
done
clear
D)
4 megasporangia on ventral surface
done
clear
View Answer play_arrow
question_answer 128) In Pinus roxburghii, the common pine tree, microsporophyll is having
A)
2 microsporangia on abaxial side of microsporophyll
done
clear
B)
4 microsporangia on abaxial side of microsporophyll
done
clear
C)
2 microsporangia on adaxial side of microsporophyll
done
clear
D)
4 microsporangia on adaxial side of microsporophyll
done
clear
View Answer play_arrow
question_answer 129) The structure, capable of stretching many times of its size, is
A)
lungs
done
clear
B)
stomach
done
clear
C)
uterus
done
clear
D)
pupil of the eye
done
clear
View Answer play_arrow
question_answer 130) The recapitulation theory or biogenetic law was proposed by
A)
Ernst Haeckel
done
clear
B)
Von Baer
done
clear
C)
Weismann
done
clear
D)
Mendel
done
clear
View Answer play_arrow
question_answer 131) The nucleolus is the site of formation of
A)
spindle fibres
done
clear
B)
chromosomes
done
clear
C)
ribosomes
done
clear
D)
peroxysomes
done
clear
View Answer play_arrow
question_answer 132) Klinefelters syndrome occurs because of
A)
presence of extra X-chromosome in male
done
clear
B)
presence of only one X-chromosome in female
done
clear
C)
presence of extra XY-chromosome in male
done
clear
D)
presence of extra Y-chromosome in male
done
clear
View Answer play_arrow
question_answer 133) RNA differs from DNA in having
A)
thymine
done
clear
B)
deoxyribose sugar
done
clear
C)
uracil
done
clear
D)
cytosine
done
clear
View Answer play_arrow
question_answer 134) Both DNA and RNA are present in
A)
bacteria
done
clear
B)
higher organisms
done
clear
C)
bacteria and higher organisms
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 135) "One gene one polypeptide chain" theory was proposed by
A)
G W Beadle and E L Tatum
done
clear
B)
Khorana and Nirenberg
done
clear
C)
Watson and Komberg
done
clear
D)
Vemon M Ingram
done
clear
View Answer play_arrow
question_answer 136) The phenomenon of heterophylly, where 2 different types of leaves are found is exhibited in which one of the following plants?
A)
Utricularia
done
clear
B)
Limnophila heterophylla
done
clear
C)
Cabomba stellaris
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 137) Ligulate leaves are found in
A)
Myrtaceae
done
clear
B)
Asteraceae
done
clear
C)
Poaceae
done
clear
D)
Cyperaceae
done
clear
View Answer play_arrow
question_answer 138) The botanical name of cauliflower is
A)
Brassica oleracea var. copitata
done
clear
B)
Brassica oleracea var. botrytis
done
clear
C)
Brassica oleracea var. gongylodis
done
clear
D)
Brassica oleracea
done
clear
View Answer play_arrow
question_answer 139) Hypanthodium inflorescence is commonly found in which one of the following plants?
A)
Ficus sp.
done
clear
B)
Brassica (cauliflower)
done
clear
C)
Polyalthia (ashok)
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 140) Reindeer moss, which is the most important and stable food for reindeer in Arctic region belongs to which group of Plantae?
A)
Algae
done
clear
B)
Bryophyta
done
clear
C)
Lichens
done
clear
D)
Pteridophyta
done
clear
View Answer play_arrow
question_answer 141) A rapid transformation from larval to adult form, in which destruction of some larval tissues and formation of adult organs occur is called
A)
metaphase
done
clear
B)
metamorphosis
done
clear
C)
metaphysic
done
clear
D)
metathorax
done
clear
View Answer play_arrow
question_answer 142) Critical state of disease is called
A)
acne
done
clear
B)
acromion
done
clear
C)
acholin
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 143) Electrostatic precipitators are extensively employed to control
A)
water pollution
done
clear
B)
air pollution
done
clear
C)
radioactive pollution
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 144) Under the umbrella of social forestry, a system has been launched, in which agriculture and forestry are involved. Select the name of the system.
A)
Block cutting system
done
clear
B)
Jhum cultivation system
done
clear
C)
Taungaya system
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 145) One of the most important raw material for paper and pulp industry comes from our vast forest resources, select the source for paper and pulp industry extensively used in our country
A)
Picea sp.
done
clear
B)
Pinus sp.
done
clear
C)
Populus sp.
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 146) Out of various resources of forest in out vast country, which one of the following is used as a basic raw material for biri industry?
A)
Diospyros cordifolia
done
clear
B)
Diospyros melanoxylon
done
clear
C)
Diospyros ebenum
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 147) Ectomycorrhiza is commonly found in
A)
Pinus
done
clear
B)
Quercus
done
clear
C)
Rhododendron
done
clear
D)
Both (a) and (b)
done
clear
View Answer play_arrow
question_answer 148) Parthenium argenatatum, which is commonly known as guayule provides, which one of the following commercial products?
A)
Para-rubber
done
clear
B)
A product equivalent to para-rubber
done
clear
C)
Chewing-gum
done
clear
D)
Jojoba oil
done
clear
View Answer play_arrow
question_answer 149) Castor oil is obtained from
A)
Acacia nilotica
done
clear
B)
Simmondsia chinensis
done
clear
C)
Ricinus communis
done
clear
D)
Both (a) and (b)
done
clear
View Answer play_arrow
question_answer 150) Select the correct botanical name of Subabul, which is extensively employed in social forestry programme as an excellent source of fast growing fuel and fodder yielding medium sized tree.
A)
Acacia nilotica
done
clear
B)
Leucas aspera
done
clear
C)
Leucaena leucocephala
done
clear
D)
Prosopis spicigera
done
clear
View Answer play_arrow
question_answer 151) If Ca of 2-celled proembryo divides longitudinally and the Ca and Cb both contribute to the development of embryo, this type of embryogeny would be called as
A)
onagrad type
done
clear
B)
asterad type
done
clear
C)
solanad type
done
clear
D)
Both (a) and (b)
done
clear
View Answer play_arrow
question_answer 152) If Ca of 2-celled proembryo divides transversely the embryogeny would be
A)
solanad and asterad type
done
clear
B)
chenopodial and onagrad type
done
clear
C)
caryophyllad and onagrad type
done
clear
D)
solanad and chenopoldial type
done
clear
View Answer play_arrow
question_answer 153) What would be the chromosome number of endosperm if it is developed from Oenothera type of embryo sac possessing chromosome number equal to 16 (haploid)?
A)
32 (diploid)
done
clear
B)
48 (triploid)
done
clear
C)
16 (haploid)
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 154) If the chromosome number of cells of nucellus of megasporangium of Pinus is 16 (sixteen), what would be the chromosome number of endosperm cells?
A)
32
done
clear
B)
16
done
clear
C)
48
done
clear
D)
08
done
clear
View Answer play_arrow
question_answer 155) Which one of the following is the most common type of embryo sac?
A)
Allium type
done
clear
B)
Oenothera type
done
clear
C)
Polygonum type
done
clear
D)
Adoxa type
done
clear
View Answer play_arrow
question_answer 156) Mucilaginous polysaccharide commonly called as carrageenin and which is extensively used in various industries is obtained from
A)
Gelidium
done
clear
B)
Laminaria
done
clear
C)
Chondrus
done
clear
D)
Gradlaria
done
clear
View Answer play_arrow
question_answer 157) Symbiotic association between fungal hyphae and the roots of Pinus is known as
A)
mycorrhiza
done
clear
B)
mycophagy
done
clear
C)
mycobiont
done
clear
D)
Both (a) and (b)
done
clear
View Answer play_arrow
question_answer 158) The characteristic cell wall material peptidoglycan has another covering of lipopolysaccharides. This specialized condition is found in
A)
eubacteria Gram positive
done
clear
B)
eubacteria Gram negative
done
clear
C)
all eukaryotes
done
clear
D)
Both (a) and (b)
done
clear
View Answer play_arrow
question_answer 159) Goldfussia in which two different size of leaves are found is an excellent example of
A)
heterophylly
done
clear
B)
anisophylly
done
clear
C)
distichous
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 160) The prickly pear plant, which is botanically named as Opuntia is an excellent example of
A)
phyllode
done
clear
B)
cladode
done
clear
C)
phylloclade
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 161) If a dry fruit is developed from bicarpellary syncarpous, inferior ovary with a single basally placed ovule, the fruit would be called as
A)
caryopsis
done
clear
B)
cypsela
done
clear
C)
capsule
done
clear
D)
siliqua
done
clear
View Answer play_arrow
question_answer 162) If a fruit is developed from (\[\overline{2G}\]), bilocular with one ovule in each locule and pendulously attached and the wall of the ovary possesses vittae, the fruit is designated as
A)
caryopsis
done
clear
B)
cypsela
done
clear
C)
cremocarp
done
clear
D)
capsule
done
clear
View Answer play_arrow
question_answer 163) If a fruit is developed from an inferior bicarpellary, syncarpous, unilocular ovary, the seed coat is free from the pericarp, the fruit would be called
A)
cypsela
done
clear
B)
silicula
done
clear
C)
siliqua
done
clear
D)
sorosis
done
clear
View Answer play_arrow
question_answer 164) If a fruit is developed from (\[\overline{2G}\]) ovary with parietal placentation, unilocular when young but becoming 2-locular with age and the appearance of the fruit so developed is pod like, the fruit would be grouped under
A)
lomentum
done
clear
B)
siliqua
done
clear
C)
silicula
done
clear
D)
capsule
done
clear
View Answer play_arrow
question_answer 165) Increasing accumulation of DDT in organisms of a food chain in higher trophic level is known as
A)
biological magnification
done
clear
B)
biotic potential
done
clear
C)
biotic component
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 166) If the anthers are fused to form a tubular structure, whereas the filaments remain free- the condition is termed as
A)
epipetalous
done
clear
B)
syngenesious
done
clear
C)
polyandrous
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 167) If the anthers are fused together forming a tubular structure while the filaments remain free, the condition is found in which one of the following family?
A)
Malvaceae
done
clear
B)
Cucurbitaceae
done
clear
C)
Solanaceae
done
clear
D)
Asteraceae
done
clear
View Answer play_arrow
question_answer 168) If both the anthers and filaments are fused together to form a compact structure with sigmoid appearance the condition is termed a
A)
syngenesious
done
clear
B)
androecious
done
clear
C)
syngynandrous
done
clear
D)
synandrous
done
clear
View Answer play_arrow
question_answer 169) The enzyme used for joining two DNA fragments is called
A)
ligase
done
clear
B)
restriction endonuclease
done
clear
C)
DNA polymerase
done
clear
D)
gyrase
done
clear
View Answer play_arrow
question_answer 170) There are certain plant species, which flower only once in their life span. This condition is named as
A)
polygamous
done
clear
B)
polycarpic
done
clear
C)
monocarpic
done
clear
D)
pericarpic
done
clear
View Answer play_arrow
question_answer 171) If an angiospermic plant produces unisexual and bisexual flowers on the same plant , the condition would be termed as
A)
dioecious
done
clear
B)
trioecious
done
clear
C)
polygamous
done
clear
D)
andro - gynoecious
done
clear
View Answer play_arrow
question_answer 172) Ligulate flowers are the diagnostic character which one of the following family?
A)
Poaceae
done
clear
B)
Asteraceae
done
clear
C)
Brassicaceae
done
clear
D)
Papilionaceae
done
clear
View Answer play_arrow
question_answer 173) In opposite phyllotaxy, if the leaves are of different size and arranged alternately, the phenomenon is termed as
A)
distichous
done
clear
B)
pentastichous
done
clear
C)
anisophylly
done
clear
D)
heterophylly
done
clear
View Answer play_arrow
question_answer 174) A funnel shaped stigma is found in
A)
Grasses
done
clear
B)
Crocus
done
clear
C)
Malvestrum
done
clear
D)
Papaver
done
clear
View Answer play_arrow
question_answer 175) A child has the blood group 0. The possible genotypes of his parents are
A)
\[{{I}^{A}}{{I}^{B}}\times {{I}^{A}}{{I}^{O}}\]
done
clear
B)
\[{{I}^{O}}{{I}^{O}}\times {{I}^{A}}{{I}^{A}}\]
done
clear
C)
\[{{I}^{A}}{{I}^{B}}\times {{I}^{O}}{{I}^{O}}\]
done
clear
D)
\[{{I}^{B}}{{I}^{O}}\times {{I}^{A}}{{I}^{O}}\]
done
clear
View Answer play_arrow
question_answer 176) Which animal has its blood composition nearly the same as of man?
A)
Gorilla
done
clear
B)
Rhesus monkey
done
clear
C)
Chimpanzee
done
clear
D)
Baboon
done
clear
View Answer play_arrow
question_answer 177) The change in the partial pressure of \[C{{O}_{2}}\]in blood is determined by
A)
carotid sinus
done
clear
B)
carotid bodies
done
clear
C)
surface of lungs
done
clear
D)
Both (a) and (b)
done
clear
View Answer play_arrow
question_answer 178) In man regulation of respiration and heart beat is maintained by
A)
cerebrum
done
clear
B)
medulla oblongata
done
clear
C)
cerebellum
done
clear
D)
spinal cord
done
clear
View Answer play_arrow
question_answer 179) The aquaduct of sylvius connects
A)
1st and 2nd ventricles
done
clear
B)
2nd and 3rd ventricles
done
clear
C)
3rd and 4th ventricles
done
clear
D)
4th ventricle and central canal
done
clear
View Answer play_arrow
question_answer 180) Which is more efficient medium for oxygen uptake in respiration?
A)
Aquatic
done
clear
B)
Aerial
done
clear
C)
Marine
done
clear
D)
Esturine
done
clear
View Answer play_arrow
question_answer 181) Fine blood capillaries seen on reddening of the eyes are spread over
A)
pupil
done
clear
B)
conjunctiva
done
clear
C)
eyelid lining
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 182) Kupffer cells are found in
A)
spleen
done
clear
B)
liver
done
clear
C)
lung alveoli
done
clear
D)
blood
done
clear
View Answer play_arrow
question_answer 183) Estimated length of blood vessels in a human is
A)
1160 km
done
clear
B)
5100 km
done
clear
C)
1080 km
done
clear
D)
80,600 km
done
clear
View Answer play_arrow
question_answer 184) Estrogen is secreted by
A)
pituitary gland
done
clear
B)
adrenal gland
done
clear
C)
Graafian follicle
done
clear
D)
placenta
done
clear
View Answer play_arrow
question_answer 185) William Harvey explained many aspects of human physiology one of them was
A)
digestive system
done
clear
B)
circulation of blood
done
clear
C)
blood group
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 186) Deficiency of vasopressin (ADH) leads to
A)
diabetes mellitus
done
clear
B)
diabetes insipidus
done
clear
C)
Graves disease
done
clear
D)
goiter
done
clear
View Answer play_arrow
question_answer 187) Body temperature of many animals corresponds to the environmental temperature. They are known as
A)
heterothermic
done
clear
B)
homoeothermic
done
clear
C)
endothermic
done
clear
D)
poikilothermic
done
clear
View Answer play_arrow
question_answer 188) In a DNA molecule
A)
nitrogenous bases are bonded covalently to phosphate groups
done
clear
B)
pentose sugars are bonded ionically to the nitrogenous bases
done
clear
C)
pentose sugars are bonded by hydrogen bonds to the nitrogenous bases
done
clear
D)
nitrogenous bases are bonded to each other by hydrogen bonds
done
clear
View Answer play_arrow
question_answer 189) Which is the wrong combination?
A)
Landsteiner - Blood group
done
clear
B)
Chargaff and Davidson - A = T, C = G
done
clear
C)
Watson and Crick - DNA structure
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 190) Cornea is covered externally by a thin transparent membrane, which is
A)
sclerotic
done
clear
B)
conjunctiva
done
clear
C)
choroid
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 191) Which one of the following is an important food element for growth and repair of tissues?
A)
Vitamin-C
done
clear
B)
Protein
done
clear
C)
Glucose
done
clear
D)
Potassium
done
clear
View Answer play_arrow
question_answer 192) The collection of various types of molecules in a cell is called
A)
primordial soup
done
clear
B)
cellular broth
done
clear
C)
cellular pool
done
clear
D)
molecular broth
done
clear
View Answer play_arrow
question_answer 193) Which one of the following is an essential fatty acid?
A)
Linoleic acid
done
clear
B)
Sulphuric acid
done
clear
C)
Nitric acid
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 194) Select the most successful group of organism on the surface of our globe
A)
Gram positive heterotrophs
done
clear
B)
Gram negative photosynthetic cyanobacteria
done
clear
C)
chemoautotrophic bacteria
done
clear
D)
photoautotrophic bacteria
done
clear
View Answer play_arrow
question_answer 195) The algal component of foliose lichen is termed as
A)
phycosymbiont
done
clear
B)
phycobiont
done
clear
C)
mycobiont
done
clear
D)
ascolichen
done
clear
View Answer play_arrow
question_answer 196) The enzyme used in the beverage industry to flavour soft drinks and in the baking industry to sweeten the biscuits and cakes
A)
glycoamylase
done
clear
B)
amylase
done
clear
C)
proteases
done
clear
D)
glucoamylase
done
clear
View Answer play_arrow
question_answer 197) Sickle cell anaemia is due to replacement of glutamic acid at the 6th position is
A)
lencine
done
clear
B)
valine
done
clear
C)
proline
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 198) A person may die after getting a bee sting in his body due to
A)
toxicity
done
clear
B)
coagulation of blood
done
clear
C)
anaphylactic shock
done
clear
D)
Both (a) and (b)
done
clear
View Answer play_arrow
question_answer 199) Colorblindness is caused by
A)
dominant gene of female
done
clear
B)
recessive gene of male
done
clear
C)
dominant gene of male
done
clear
D)
recessive gene of female
done
clear
View Answer play_arrow
question_answer 200) An antigen in the white blood corpuscles of man is known as
A)
HIV
done
clear
B)
HLA
done
clear
C)
AHL
done
clear
D)
LAH
done
clear
View Answer play_arrow
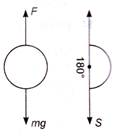 -730 J
-730 J 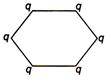
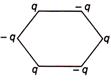
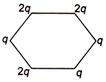
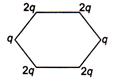
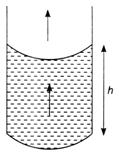 The thickness of wall of capillary is negligible. (surface tension of water \[=\text{ 73}.\text{5}\times \text{1}{{0}^{-3}}\text{ N}/\text{m},\] density of water \[\text{1}{{0}^{17}}\]
The thickness of wall of capillary is negligible. (surface tension of water \[=\text{ 73}.\text{5}\times \text{1}{{0}^{-3}}\text{ N}/\text{m},\] density of water \[\text{1}{{0}^{17}}\]