A) \[{{P}_{3}}>{{P}_{1}},W>0\]
B) \[{{P}_{3}}>{{P}_{1}},W<0\]
C) \[{{P}_{3}}>{{P}_{1}},W<0\]
D) \[{{P}_{3}}={{P}_{1}},W=0\]
Correct Answer: C
Solution :
Slope of adiabatic process at a given state (P,V,T) is more than the slope of isothermal process. The corresponding P-V graph for the two processes is as shown in figure.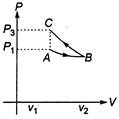 In the graph, AB is isothermal and BC is adiabatic. \[{{W}_{AB}}=\]positive (as volume is increasing) and\[{{W}_{BC}}=\]negative (as volume is decreasing) \[|{{W}_{BC}}|\,>\,|{{W}_{AB}}|,\]as area under P-V graph gives the work done. Hence, \[{{W}_{AB}}+{{W}_{BC}}=W<0\] From the graph itself, it is clear that \[{{p}_{3}}>{{p}_{1}}\]
In the graph, AB is isothermal and BC is adiabatic. \[{{W}_{AB}}=\]positive (as volume is increasing) and\[{{W}_{BC}}=\]negative (as volume is decreasing) \[|{{W}_{BC}}|\,>\,|{{W}_{AB}}|,\]as area under P-V graph gives the work done. Hence, \[{{W}_{AB}}+{{W}_{BC}}=W<0\] From the graph itself, it is clear that \[{{p}_{3}}>{{p}_{1}}\]
You need to login to perform this action.
You will be redirected in
3 sec
