A) 2 and 2
B) 0 and 2
C) 0 and 1
D) 0 and 0
Correct Answer: D
Solution :
[d] \[[M{{A}_{2}}{{B}_{2}}]\]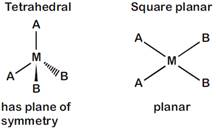 Total number of optical isomer is zero in both the cases.
Total number of optical isomer is zero in both the cases.
You need to login to perform this action.
You will be redirected in
3 sec
