A) \[{{T}_{f}}=\frac{3}{7}{{T}_{0}}\]
B) \[{{T}_{f}}=\frac{7}{3}{{T}_{0}}\]
C) \[{{T}_{f}}=\frac{3}{2}{{T}_{0}}\]
D) \[{{T}_{f}}=\frac{5}{2}{{T}_{0}}\]
Correct Answer: C
Solution :
| [c] Here, change in internal energy of the system is zero. i.e., increase in internal energy of one is equal to decrease in internal energy of other. |
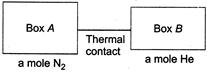 |
| |
| |
| Now, |
| |
| |
| |
| |
You need to login to perform this action.
You will be redirected in
3 sec
