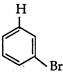
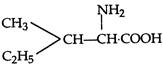
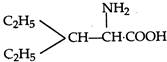
question_answer 1) Two spherical shells one which is placed in air has constant charge \[{{Q}_{1}}.\] and the other which is placed in a medium of dielectric constant K has a charge \[{{Q}_{2}}.\] The ratio of forces between the shells is:
A)
\[K:1\]
done
clear
B)
\[1:K\]
done
clear
C)
\[{{K}^{2}}:1\]
done
clear
D)
\[1:{{K}^{2}}\]
done
clear
View Answer play_arrow
question_answer 2) A particle of unit mass and specific charge s is thrown from the wall perpendicularly to a wall at a distance d from the wall with speed \['\upsilon '.\] The minimum magnetic field produced so that the particle does not touch the wall, is:
A)
\[\frac{\upsilon }{sd}\]
done
clear
B)
\[\frac{2\upsilon }{sd}\]
done
clear
C)
\[\frac{\upsilon }{2sd}\]
done
clear
D)
\[\frac{\upsilon }{4sd}\]
done
clear
View Answer play_arrow
question_answer 3) A block of mass 1 kg is placed on the wall under a force F. If coefficient of L friction \[(\mu )\] is 0.5, then minimum value F of force applied is :
A)
9.8 N
done
clear
B)
49 N
done
clear
C)
19.6 N
done
clear
D)
1.96 N
done
clear
View Answer play_arrow
question_answer 4) Mass of a planet is 10 times that of earth and radius is 2 times that of earth. If at the earth, the escape velocity of a satellite is \[{{\upsilon }_{es(e),}}\] then escape velocity at the planet is :
A)
\[\sqrt{5}\,\,{{\upsilon }_{es(e)}}\]
done
clear
B)
\[5\,\,{{\upsilon }_{es(e)}}\]
done
clear
C)
\[2\sqrt{5}\,\,{{\upsilon }_{es(e)}}\]
done
clear
D)
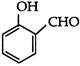
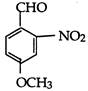
\[10\,\,{{\upsilon }_{es(e)}}\]
done
clear
View Answer play_arrow
question_answer 5) An electron beam is accelerated through a potential difference of V volt. The minimum wavelength of X-rays produced is :
A)
\[\frac{he}{cV}\]
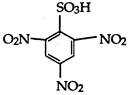
done
clear
B)
\[\frac{cV}{he}\]
done
clear
C)
\[\frac{eV}{hc}\]
done
clear
D)
\[\frac{hc}{eV}\]
done
clear
View Answer play_arrow
question_answer 6) Light waves of wavelength \[\lambda \] is incident on a metal of work function \[\theta \] Maximum velocity of the electron is :
A)
\[{{\left[ \frac{2(h\lambda -\theta )}{m} \right]}^{1/2}}\]
done
clear
B)
\[{{\left[ \frac{2(h\lambda -\lambda \theta )}{m\lambda } \right]}^{1/2}}\]
done
clear
C)
\[\frac{2(hc-\lambda \theta )}{m}\]
done
clear
D)
\[\left[ \frac{hc+\lambda \theta }{m\lambda } \right]\]
done
clear
View Answer play_arrow
question_answer 7) A car and a container, moving with equal kinetic energies are stopped by applying a negative acceleration. Then
A)
container will move less distance before being stopped
done
clear
B)
both will become stationary after moving some distance
done
clear
C)
car will move less distance before being stopped
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 8) Eight small drops, each of equal charges and radii are coalesce to form a big drop. The capacity of bigger drop in comparison to each smaller drop will be :
A)
4 times
done
clear
B)
2 times
done
clear
C)
32 times
done
clear
D)
8 times
done
clear
View Answer play_arrow
question_answer 9) An organ pipe, opened at both ends, has fundamental frequency of 30 Hz. If one of its ends is closed/ then fundamental frequency will be :
A)
15 Hz
done
clear
B)
30 Hz
done
clear
C)
45 Hz
done
clear
D)
60 Hz
done
clear
View Answer play_arrow
question_answer 10) A charged particle is moving in a uniform magnetic field in a circular path of radius R. When energy of the particle is doubled, then the new radius will be :
A)
\[R\]
done
clear
B)
\[R\sqrt{2}\]
done
clear
C)
\[R/\sqrt{2}\]
done
clear
D)
\[2R\]
done
clear
View Answer play_arrow
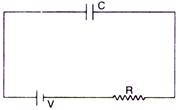
question_answer 11)
A capacitor of capacity C is charged by connecting it with R according to the figure. Energy supplied by the battery in this process is :
A)
more than \[\frac{1}{2}C{{V}^{2}}\]
done
clear
B)
\[\frac{1}{2}C{{V}^{2}}\]
done
clear
C)
\[2C{{V}^{2}}\]
done
clear
D)
less than\[\frac{1}{2}C{{V}^{2}}\]
done
clear
View Answer play_arrow
question_answer 12) If a current is flowing in a spring, then it:
A)
compress
done
clear
B)
swing
done
clear
C)
expand
done
clear
D)
remain unaffected
done
clear
View Answer play_arrow
question_answer 13) The mass of a solid sphere is 2 kg and radius is 10 cm. Sphere, tied to one end of 1 m long string, is rotated at a rate of 10rad/sec. The rotational kinetic energy of sphere is :
A)
121.4 J
done
clear
B)
185J
done
clear
C)
15.2 J
done
clear
D)
22.0 J
done
clear
View Answer play_arrow
question_answer 14) A rocket is accelerated with speed \[\upsilon =2\sqrt{g{{R}_{e}}}\] near earth surface and then it moves upward. At far distance from the earth surface, the speed of the rocket will be:
A)
\[\sqrt{g{{R}_{e}}/2}\]
done
clear
B)
\[\sqrt{g{{R}_{e}}}\]
done
clear
C)
\[(2-\sqrt{2})\sqrt{g{{R}_{e}}}\]
done
clear
D)
\[\sqrt{2g{{R}_{e}}}\]
done
clear
View Answer play_arrow
question_answer 15) In the rotational motion, different particles of the body have :
A)
both linear and angular velocities equal
done
clear
B)
linear velocities equal but different angular velocities
done
clear
C)
angular velocities equal but different linear velocities
done
clear
D)
both linear and angular velocity different
done
clear
View Answer play_arrow
question_answer 16) Two bulbs A and B are connected in parallel. Bulb A will glow more than bulb B. If their resistances are \[{{R}_{A}}\] and \[{{R}_{A}}\] respectively. Then :
A)
\[{{R}_{A}}<{{R}_{B}}\]
done
clear
B)
\[{{R}_{A}}={{R}_{B}}\]
done
clear
C)
\[{{R}_{A}}>{{R}_{B}}\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 17) A body has initial temperature of 80°C. It cools by radiation process. In 5 minutes, it cools down to 64°C and in 10 minutes, it cools down to 52°C. The temperature of surrounding is:
A)
8°C
done
clear
B)
16°C
done
clear
C)
32°C
done
clear
D)
24°C
done
clear
View Answer play_arrow
question_answer 18) The surface tension of the soap solution is T. The work done in forming a soap bubble of radius r, is:
A)
\[8\pi {{r}^{2}}T\]
done
clear
B)
\[4\pi {{r}^{2}}T\]
done
clear
C)
\[\frac{4}{3}\pi {{r}^{2}}T\]
done
clear
D)
\[\frac{8}{3}\pi {{r}^{2}}T\]
done
clear
View Answer play_arrow
question_answer 19) A radioactive substance containing N atoms emits \[n\,\alpha \]-particles per second, Half-life of the substance is :
A)
\[\frac{N}{n}\sec \]
done
clear
B)
\[\frac{n}{N}\sec \]
done
clear
C)
\[\frac{0.693n}{N}\sec \]
done
clear
D)
\[\frac{0.693N}{n}\sec \]
done
clear
View Answer play_arrow
question_answer 20) A car covers 2/5 of a certain distance with speed \[{{\upsilon }_{1}}\] and rest 3/5 part with velocity \[{{\upsilon }_{2.}}\] The average speed of the car is:
A)
\[\frac{1}{2}\sqrt{{{\upsilon }_{1}}{{\upsilon }_{2}}}\]
done
clear
B)
\[\frac{5{{\upsilon }_{1}}{{\upsilon }_{1}}}{3{{\upsilon }_{1}}+2{{\upsilon }_{2}}}\]
done
clear
C)
\[\frac{2{{\upsilon }_{1}}{{\upsilon }_{2}}}{{{\upsilon }_{1}}+{{\upsilon }_{2}}}\]
done
clear
D)
\[\frac{{{\upsilon }_{1}}+{{\upsilon }_{2}}}{2}\]
done
clear
View Answer play_arrow
question_answer 21) If the temperature of a black body is doubled, the wavelength at which the spectral radiancy has its maximum, is :
A)
doubled
done
clear
B)
halved
done
clear
C)
quadrupled
done
clear
D)
unchanged
done
clear
View Answer play_arrow
question_answer 22) A light wave travels from glass to water. The refractive indices for glass and water are\[\frac{3}{2}\]and\[\frac{4}{3}\] respectively. The value of the critical angle will be:
A)
\[{{\sin }^{-1}}\left( \frac{1}{2} \right)\]
done
clear
B)
\[{{\sin }^{-1}}\left( \frac{9}{8} \right)\]
done
clear
C)
\[{{\sin }^{-1}}\left( \frac{9}{8} \right)\]
done
clear
D)
\[{{\sin }^{-1}}\left( \frac{5}{7} \right)\]
done
clear
View Answer play_arrow
question_answer 23) In a radioactive decay \[^{92}{{U}_{238}}\xrightarrow{\alpha }X\xrightarrow{\alpha }z{{Y}^{A}}\]The values of Z and A:
A)
92, 236
done
clear
B)
88, 230
done
clear
C)
90, 130
done
clear
D)
88, 234
done
clear
View Answer play_arrow
question_answer 24) Magnetic and electric field are acting on a proton moving with velocity \[\upsilon .\] Proton will be undefeated if:
A)
E is parallel to \[\upsilon .\] but perpendicular to B
done
clear
B)
E and B both are parallel to \[\upsilon .\]
done
clear
C)
E and B are perpendicular to each other
done
clear
D)
E B and v are perpendicular to one another and \[\upsilon =\frac{E}{B}\]
done
clear
View Answer play_arrow
question_answer 25) The unit of permittivity \[({{\varepsilon }_{0}})\] of space- is :
A)
newton-metre 2 /coulomb2
done
clear
B)
coulomb/newton-metre
done
clear
C)
coulomb/newton-metre2
done
clear
D)
coulomb/newton-metre2
done
clear
View Answer play_arrow
question_answer 26) Two charges each of equal magnitude \[3.2\times {{10}^{-19}}\]coulomb but of opposite sign form an electric dipole. The distance between the two charges is 2.4 Å. If the dipole is placed in an electric field of \[5\times {{10}^{5}}\]volt/metre, then in equilibrium its potential energy will be :
A)
\[3\times {{15}^{-23}}\]joule
done
clear
B)
\[-3.84\times {{10}^{-23}}\]joule
done
clear
C)
\[-6\times {{W}^{23}}\]joule
done
clear
D)
\[-2\times {{10}^{-}}^{26}\]joule
done
clear
View Answer play_arrow
question_answer 27) The wavelength of light coming from a distant galaxy is found to be 0.5% greater than the wavelength of light coming from a stationary source. The galaxy is :
A)
coming towards earth with velocity of light
done
clear
B)
moving away from earth with velocity \[1.5\times {{10}^{6}}m/sec\]
done
clear
C)
stationary with respect to earth
done
clear
D)
moving away from earth with velocity of light
done
clear
View Answer play_arrow
question_answer 28) A convex lens of focal length 12 cm is made up of a glass of refractive index \[\frac{3}{2.}\]When it is immersed in a liquid of refractive index\[\frac{5}{4.}\] its focal length will be :
A)
15 cm
done
clear
B)
6 cm
done
clear
C)
30 cm
done
clear
D)
24 cm
done
clear
View Answer play_arrow
question_answer 29) When a \[p-n\] junction diode is connected to a battery with its p-side to positive terminal and n-side to negative terminal, the width of the depletion layer:
A)
decreases
done
clear
B)
in creases
done
clear
C)
first decreases then increases
done
clear
D)
first increases then decreases
done
clear
View Answer play_arrow
question_answer 30) Wave nature of light does not explain :
A)
Interference
done
clear
B)
Total internal reflection
done
clear
C)
Photo-electric effect
done
clear
D)
Refraction
done
clear
View Answer play_arrow
question_answer 31) A resistance of 8 ohm and an inductance of 6 ohm are connected in series to an A.C. circuit. The impedance of the circuit is ;
A)
\[14\sqrt{2}\Omega \]
done
clear
B)
\[10\Omega \]
done
clear
C)
\[2\Omega \]
done
clear
D)
\[15\Omega \]
done
clear
View Answer play_arrow
question_answer 32) A mixture of oxygen and hydrogen are filled in a vessel at a fixed temperature. The ratio of average kinetic energies of atoms of H2 and O2 is :
A)
1 : 16
done
clear
B)
1 : 8
done
clear
C)
1 : 12
done
clear
D)
1 : 1
done
clear
View Answer play_arrow
question_answer 33) In forward and reverse biasing of a \[p-n\] junction diode, the ratio of resistances is :
A)
102 : 1
done
clear
B)
10-2:1
done
clear
C)
1:104
done
clear
D)
1: 10-4
done
clear
View Answer play_arrow
question_answer 34) Lenz's law is based on the principle of conservation of :
A)
mass
done
clear
B)
charge
done
clear
C)
momentum
done
clear
D)
energy
done
clear
View Answer play_arrow
question_answer 35) An ice at -50°C is to be converted into vapour at 100°C. The heat required in this process is : (specific heat of ice = 0.5 calorie/gram 0C Latent heat of ice = 80 calorie/gram Latent heat of vapour =540 calorie/gram)
A)
1028 ealorie
done
clear
B)
624 calorie
done
clear
C)
745 calorie
done
clear
D)
810 calorie
done
clear
View Answer play_arrow
question_answer 36) The speed of sound in a gas \[(\gamma -1.5)\] at 300 K is 340 m/s. The rms velocity of gas molecules will be
A)
340 m/s
done
clear
B)
\[\frac{340}{\sqrt{2}}\]m/s
done
clear
C)
340 \[\sqrt{2}\] m/s
done
clear
D)
340\[\sqrt{15}\] m/s
done
clear
View Answer play_arrow
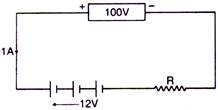
question_answer 37)
A battery of 12 volt is charged by a supply of 100 volt as shown in the figure. The charging current is 1.0A. Calculate the value of R :
A)
\[45\Omega \]
done
clear
B)
\[105\,\Omega \]
done
clear
C)
\[88\,\Omega \]
done
clear
D)
\[95\,\Omega \]
done
clear
View Answer play_arrow
question_answer 38) In a sample of radioactive substance, what percentage decays in one mean life time?
A)
69.3%
done
clear
B)
63%
done
clear
C)
50%
done
clear
D)
36%
done
clear
View Answer play_arrow
question_answer 39) At an instant a positive charge is moving parallel to a current carrying conductor. The path of the positive charge is :
A)
elliptical
done
clear
B)
circle
done
clear
C)
straight line
done
clear
D)
parabola
done
clear
View Answer play_arrow
question_answer 40) The momentum of two masses \[{{m}_{1}}\]and \[{{m}_{2}}\]are same. The ratio of their kinetic energies \[{{E}_{1}}\] and \[{{E}_{2}}\] is:
A)
\[\sqrt{{{m}_{1}}}:\sqrt{{{m}_{2}}}\]
done
clear
B)
\[{{m}_{1}}:{{m}_{2}}\]
done
clear
C)
\[{{m}_{2}}:{{m}_{1}}\]
done
clear
D)
\[m_{1}^{2}:m_{2}^{2}\]
done
clear
View Answer play_arrow
question_answer 41) A current of 0.1 ampere is flowing through a coil of 1000 turns and radius metre. 0.1 The magnetic field at the centre of the coil is :
A)
\[2\times {{10}^{-1}}T\]
done
clear
B)
\[4.31\times {{10}^{-2}}T\]
done
clear
C)
\[6.28\times {{10}^{-4}}T\]
done
clear
D)
\[9.81\times {{10}^{-4}}T\]
done
clear
View Answer play_arrow
question_answer 42) If 2 g mole of diatomic gas and 1 g mole of monoatomic gas are mixed together, then the ratio of specific heats of mixture is:
A)
1.24
done
clear
B)
1.58
done
clear
C)
1.46
done
clear
D)
1.60
done
clear
View Answer play_arrow
question_answer 43) A p-type semiconductor is formed by adding 1 indium atom in to a sample of silicon per \[5\times {{10}^{-7}}\] silicon atoms. If the density of atoms in the sample is \[5\times {{10}^{28}}\] atoms/metre3, the number of atoms accepted in per metre of silicon is :
A)
\[1.0\times {{10}^{13}}atoms/c{{m}^{3}}\]
done
clear
B)
\[2.5\times {{10}^{20}}atoms/c{{m}^{3}}\]
done
clear
C)
\[2.5\times 10atoms/c{{m}^{3}}\]
done
clear
D)
\[1.0\times {{10}^{15}}atoms/c{{m}^{3}}\]
done
clear
View Answer play_arrow
question_answer 44) At a certain place, magnitude of horizontal and vertical components of a magnetic field are same. The total intensity of magnetic field at that place will be :
A)
H
done
clear
B)
H2
done
clear
C)
2H
done
clear
D)
\[\sqrt{2}H\]
done
clear
View Answer play_arrow
question_answer 45) A coil having resistance of 50 ohm and an inductance of 10 ohm, is connected to a 100 volt battery. The energy stored in the coil is :
A)
10J
done
clear
B)
20J
done
clear
C)
30 J
done
clear
D)
40 J
done
clear
View Answer play_arrow
question_answer 46) The ratio of intensities of two waves is 1: 9.The waves interfere, what will be the ratio of maximum and minimum intensities?
A)
1 : 4
done
clear
B)
4 : 1
done
clear
C)
3 : 2
done
clear
D)
2 : 3
done
clear
View Answer play_arrow
question_answer 47) A capacitor when charged by a potential difference of 200 volt, stores a charge of 0.1 C. By discharging, energy liberated by the capacitor is :
A)
-30 J
done
clear
B)
-15 J
done
clear
C)
10 J
done
clear
D)
20J
done
clear
View Answer play_arrow
question_answer 48) A small current carrying loop acts as a small magnet. The area of the loop is A and magnetic moment is M. The current in the loop will be :
A)
\[\frac{A}{M}\]
done
clear
B)
\[\frac{M}{A}\]
done
clear
C)
\[MA\]
done
clear
D)
\[{{A}^{2}}M\]
done
clear
View Answer play_arrow
question_answer 49) The core used in transformer and other electromagnetic instruments is laminated, the reason is :
A)
to increase the electric field
done
clear
B)
to increase the magnetic field
done
clear
C)
(c) to reduce the loss of energy due to eddy currents
done
clear
D)
to decrease the resistance
done
clear
View Answer play_arrow
question_answer 50) The light of frequency v falls on a certain photoelectric substance of threshold frequency \[{{v}_{0}}.\] The work function of the substance is :
A)
\[h{{v}_{0}}\]
done
clear
B)
\[hv\]
done
clear
C)
\[\frac{h\gamma }{{{v}_{0}}}\]
done
clear
D)
\[h(v-{{v}_{0}})\]
done
clear
View Answer play_arrow
question_answer 51) The magnification of an astronomical telescope is 10 and the focal length of eye-piece is 20 cm. The focal length of objective lens is :
A)
\[\frac{1}{200}\] cm
done
clear
B)
2 cm
done
clear
C)
200 cm
done
clear
D)
100 cm
done
clear
View Answer play_arrow
question_answer 52) A car is moving with speed 30 m/s on a circular path of radius 500 m. If its speed is increasing at a rate of \[5m/{{s}^{2}},\] then resultant acceleration at that instant is :
A)
6.83 m/s2
done
clear
B)
8 m/s2
done
clear
C)
5.31 m/s2
done
clear
D)
4 m/s2
done
clear
View Answer play_arrow
question_answer 53) Apparent frequency of sound of engine is changing in the ratio 5/3 under the condition that engine is first approaching and then receding away from the observer. If velocity of sound is 340 m/s, then velocity of engine is :
A)
340 m/s
done
clear
B)
170 m/s
done
clear
C)
85 m/s
done
clear
D)
310 m/s
done
clear
View Answer play_arrow
question_answer 54) A diver, at a depth of 12 metre from the surface of the water, sees the sky with in a cone whose half vertex angle \[\left( _{a}{{\mu }_{\omega =}}\frac{4}{3} \right)\]is :
A)
\[\cos -\frac{4}{3}\]
done
clear
B)
\[{{\sin }^{-1}}\frac{4}{3}\]
done
clear
C)
\[{{90}^{0}}\]
done
clear
D)
\[{{\sin }^{-1}}\frac{3}{4}\]
done
clear
View Answer play_arrow
question_answer 55) In a galvanometer of 5 ohm resistance, a wire of 2 ohm resistance is used as a shunt. If the total current is 1 ampere, the fraction of current that will flow through the shunt is :
A)
1.2 A
done
clear
B)
0.7A
done
clear
C)
0.5 A
done
clear
D)
0.3 A
done
clear
View Answer play_arrow
question_answer 56) 1 newton/coulomb is equivalent to :
A)
1 C/V
done
clear
B)
1 J
done
clear
C)
1 V/M
done
clear
D)
1 J/C
done
clear
View Answer play_arrow
question_answer 57) An electron is moving with velocity \[\upsilon \] on a circular path of radius r in a transverse electric field B. The specific charge (elm) of the electron is:
A)
\[B\upsilon r\]
done
clear
B)
\[\frac{\upsilon }{Br}\]
done
clear
C)
\[B\upsilon {{r}^{2}}\]
done
clear
D)
\[\frac{B}{r\upsilon }\]
done
clear
View Answer play_arrow
question_answer 58) The false statement is :
A)
Every SHM is periodic in nature
done
clear
B)
Every periodic motion is SHM
done
clear
C)
In every SHM, total energy is proportional to the square of amplitude
done
clear
D)
In SHM, acceleration of oscillating body is proportional to the displacement from the equilibrium position
done
clear
View Answer play_arrow
question_answer 59) n balls each of mass m and velocity \[\upsilon \] collide with a wall, assume that the collisions are perfectly elastic. The a pressure exerted per second on the well is:
A)
3 \[mn\upsilon \]
done
clear
B)
\[mn\upsilon \]
done
clear
C)
\[2mn\upsilon \]
done
clear
D)
none
done
clear
View Answer play_arrow
question_answer 60) Number of extra electrons in a body of charge \[-\text{ }80\,\mu C,\] is :
A)
\[80\times {{10}^{15}}\]
done
clear
B)
\[80\times {{10}^{-}}^{15}\]
done
clear
C)
\[5\times {{10}^{14}}\]
done
clear
D)
\[1.28\times {{10}^{-}}^{17}\]
done
clear
View Answer play_arrow
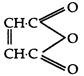
question_answer 61) What is obtained, when propene is treated with N-bromo sucdnimide?
A)
\[C{{H}_{3}}-\underset{\begin{smallmatrix} | \\ Br \end{smallmatrix}}{\mathop{C}}\,=C{{H}_{2}}\]
done
clear
B)
\[BrC{{H}_{2}}CH=C{{H}_{2}}\]
done
clear
C)
\[BrC{{H}_{2}}CH=CHBr\]
done
clear
D)
\[BrC{{H}_{2}}\underset{\begin{smallmatrix} | \\ Br \end{smallmatrix}}{\mathop{CH}}\,=CHBr\]
done
clear
View Answer play_arrow
question_answer 62) Poisonous gas 'Lewisite' is obtained by the reaction of:
A)
\[CH\equiv CH\]and \[AsC{{l}_{3}}\]
done
clear
B)
\[C{{H}_{2}}=C{{H}_{2}}\]and\[AsC{{l}_{3}}\]
done
clear
C)
\[CH\equiv CH\] and\[{{S}_{2}}C{{l}_{2}}\]
done
clear
D)
\[C{{H}_{2}}=C{{H}_{2}}\]and\[NOCl\]
done
clear
View Answer play_arrow
question_answer 63) Phenol, in Liebermann's nitroso test, give the colour:
A)
red
done
clear
B)
yellow
done
clear
C)
blue
done
clear
D)
pink
done
clear
View Answer play_arrow
question_answer 64) What will be the main product, when benzene vapours are passed over\[{{V}_{2}}{{O}_{5}}\]at\[450{}^\circ C\]?
A)
\[\underset{CH.COOH}{\overset{CH.COOH}{\mathop{|\,|}}}\,\]
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 65) Which is the best reagent to distinguish between propene and propyne?
A)
ammonical\[AgN{{O}_{3}}\]
done
clear
B)
\[\frac{B{{r}_{2}}}{CC{{l}_{4}}}\]
done
clear
C)
alkaline\[KMn{{O}_{4}}\]
done
clear
D)
aqueous\[B{{r}_{2}}\]
done
clear
View Answer play_arrow
question_answer 66) The octane number of a sample of petrol is 40. It means that its knocking property is equal to the mixture of:
A)
40% n-heptane + 60% iso-octane
done
clear
B)
40% petrol + 60% iso-octane
done
clear
C)
60% n-heptane + 40% iso-octane
done
clear
D)
60% petrol + 40% iso-octane
done
clear
View Answer play_arrow
question_answer 67) What will be the product, when carboxy phenol, obtained by Reimer Tiemann's process, is deoxidised with Zn powder?
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 68)
What is the correct IUPAC name of:
A)
4-methoxy-2-nitrobenzaldehyde
done
clear
B)
4-fonnyl-3-nitro anisole
done
clear
C)
4-mefhoxy-6-nitrobenzaldehyde
done
clear
D)
2-formyl-5-methoxy nitrobenzene
done
clear
View Answer play_arrow
question_answer 69) The safest and the most common alternative of sugar is:
A)
glucose
done
clear
B)
aspartame
done
clear
C)
saccharin
done
clear
D)
cyclodextrin
done
clear
View Answer play_arrow
question_answer 70) The main component of marsh gas is:
A)
methane
done
clear
B)
ethane
done
clear
C)
butane
done
clear
D)
isobutene
done
clear
View Answer play_arrow
question_answer 71) The reaction, \[C{{H}_{3}}-\overset{\begin{smallmatrix} O \\ || \end{smallmatrix}}{\mathop{C}}\,-OC{{H}_{3}}+{{C}_{2}}{{H}_{5}}OH\xrightarrow{{{H}^{+}}or\,O{{H}^{-}}}\] \[C{{H}_{3}}-\overset{\begin{smallmatrix} O \\ || \end{smallmatrix}}{\mathop{C}}\,-O{{C}_{2}}{{H}_{5}}+C{{H}_{3}}OH\]is called:
A)
Perkin's reaction
done
clear
B)
Claisen Schmidt reaction
done
clear
C)
Esterification
done
clear
D)
Trans-esterification
done
clear
View Answer play_arrow
question_answer 72) Which one of these elements is found in vitamin\[{{B}_{12}}\]?
A)
Mg
done
clear
B)
Fe
done
clear
C)
Zn
done
clear
D)
Co
done
clear
View Answer play_arrow
question_answer 73) The monomer of teflon polymer is:
A)
\[C{{F}_{2}}=C{{F}_{2}}\]
done
clear
B)
\[C{{F}_{2}}=CC{{l}_{2}}\]
done
clear
C)
\[C{{H}_{2}}FC{{H}_{2}}F\]
done
clear
D)
\[C{{F}_{2}}=CFCl\]
done
clear
View Answer play_arrow
question_answer 74) The product obtained, heating ethanol with cone.\[{{H}_{2}}S{{O}_{4}}\]at\[165{}^\circ -170{}^\circ C,\]is:
A)
\[{{({{C}_{2}}{{H}_{5}})}_{2}}S{{O}_{4}}\]
done
clear
B)
\[C{{H}_{2}}=C{{H}_{2}}\]
done
clear
C)
\[C{{H}_{3}}COOH\]
done
clear
D)
\[{{C}_{2}}{{H}_{5}}HS{{O}_{4}}\]
done
clear
View Answer play_arrow
question_answer 75) The structural formula of picric acid is:
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 76) The reaction,\[2C{{H}_{3}}-\underset{\begin{smallmatrix} |\,| \\ O \end{smallmatrix}}{\mathop{C}}\,-O{{C}_{2}}{{H}_{5}}\xrightarrow[{}]{{{C}_{2}}{{H}_{5}}ONa}\]\[C{{H}_{3}}-\underset{\begin{smallmatrix} |\,| \\ O \end{smallmatrix}}{\mathop{C}}\,-C{{H}_{2}}-\underset{\begin{smallmatrix} |\,| \\ O \end{smallmatrix}}{\mathop{C}}\,-O{{C}_{2}}{{H}_{5}}+{{C}_{2}}{{H}_{5}}OH\]is called:
A)
Etard reaction
done
clear
B)
Perkin's reaction
done
clear
C)
Claisen condensation
done
clear
D)
Claisen Schmidt reaction
done
clear
View Answer play_arrow
question_answer 77) Formaldehyde reacts with ammonia to give urotropine. The formula of urotropine is:
A)
\[{{(C{{H}_{2}})}_{6}}{{N}_{4}}\]
done
clear
B)
\[{{(C{{H}_{2}})}_{4}}{{N}_{3}}\]
done
clear
C)
\[{{(C{{H}_{2}})}_{6}}{{N}_{6}}\]
done
clear
D)
\[{{(CH{{ }_{3}})}_{3}}{{N}_{3}}\]
done
clear
View Answer play_arrow
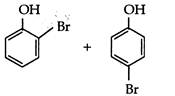
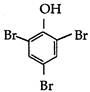
question_answer 78) Phenol reacts with aqueous bromine to give:
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 79) Which equation is correct for first order reactions?
A)
\[{{t}_{1/2}}\propto {{C}^{-1}}\]
done
clear
B)
\[{{t}_{1/2}}\propto C\]
done
clear
C)
\[{{t}_{1/2}}\propto {{C}^{0}}\]
done
clear
D)
\[{{t}_{1/2}}\propto {{C}^{1/2}}\]
done
clear
View Answer play_arrow
question_answer 80) How many neutrons are there in nucleus 'X', if it gives\[_{7}{{N}^{14}}\]after two successive \[\beta -\]particle emissions?
A)
7
done
clear
B)
10
done
clear
C)
14
done
clear
D)
9
done
clear
View Answer play_arrow
question_answer 81) 87.5% decomposition of a radioactive substance complete in 3 hours. What is the half-life of that substance?
A)
2 hours
done
clear
B)
3 hours
done
clear
C)
90 minutes
done
clear
D)
1 hours
done
clear
View Answer play_arrow
question_answer 82) 2.0 molar solution is obtained, when 0.5 mole solute is dissolved in:
A)
250 ml solvent
done
clear
B)
250 g solvent
done
clear
C)
250 ml solution
done
clear
D)
1000 ml solvent
done
clear
View Answer play_arrow
question_answer 83) The energy equivalent to 1 amu., is:
A)
931.1 MeV
done
clear
B)
\[1.492\times {{10}^{13}}erg\]
done
clear
C)
1000 J
done
clear
D)
107 erg
done
clear
View Answer play_arrow
question_answer 84) The specific rotation of equilibrium mixture of \[\alpha -\]D-glucose and\[\beta -\] D-glucose, is:
A)
\[+19{}^\circ \]
done
clear
B)
\[+112{}^\circ \]
done
clear
C)
\[+52{}^\circ \]
done
clear
D)
\[+100{}^\circ \]
done
clear
View Answer play_arrow
question_answer 85) The ratio of\[F{{e}_{2}}{{O}_{3}}\]and Al, in thermite is:
A)
1 : 3
done
clear
B)
1 : 2
done
clear
C)
3 : 1
done
clear
D)
none of these
done
clear
View Answer play_arrow
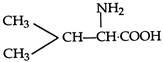
question_answer 86) The structural formula of an amino acid, isoleucine is:
A)
\[C{{H}_{3}}-\overset{\begin{smallmatrix} N{{H}_{2}} \\ | \end{smallmatrix}}{\mathop{CH}}\,.COOH\]
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 87) Which particle can be used to change\[_{13}A{{l}^{27}}\]into is\[_{15}{{P}^{30}}\]?
A)
Neutron
done
clear
B)
\[\alpha -\]particle
done
clear
C)
Proton
done
clear
D)
Deuteron
done
clear
View Answer play_arrow
question_answer 88) Which of the following reaction is catalysed by enzyme maltase?
A)
\[starch\xrightarrow{{}}maltose\]
done
clear
B)
\[maltose\xrightarrow{{}}glucose\]
done
clear
C)
\[lactose\xrightarrow[{}]{{}}maltose\]
done
clear
D)
\[maltose\xrightarrow{{}}glucose+fructose\]
done
clear
View Answer play_arrow
question_answer 89) If equilibrium constant for reaction\[2AB~{{A}_{2}}+{{B}_{2}},\]is 49, then the equilibrium constant for reaction\[AB\frac{1}{2}{{A}_{2}}+\frac{1}{2}{{B}_{2}},\]will be;
A)
7
done
clear
B)
280
done
clear
C)
49
done
clear
D)
0.01
done
clear
View Answer play_arrow
question_answer 90) The components of Zeigler Natta catalyst, used in the polymerisation of propylene, are:
A)
\[TiC{{l}_{3}}+Al{{({{C}_{2}}{{H}_{5}})}_{3}}\]
done
clear
B)
\[TiC{{l}_{4}}+Al{{({{C}_{2}}{{H}_{5}})}_{3}}\]
done
clear
C)
\[Ti{{({{C}_{2}}{{H}_{5}})}_{3}}+AlC{{l}_{3}}\]
done
clear
D)
\[Ti{{({{C}_{2}}{{H}_{5}})}_{4}}+AlC{{l}_{3}}\]
done
clear
View Answer play_arrow
question_answer 91) What will be the normality of a solution, containing 4.9 g.\[{{H}_{3}}P{{O}_{4}}\]dissolved in 500 ml water?
A)
0.3
done
clear
B)
1.0
done
clear
C)
3.0
done
clear
D)
0.1
done
clear
View Answer play_arrow
question_answer 92) The cyanohydrin of which compound, gives lactic acid on hydrolysis?
A)
\[HCHO\]
done
clear
B)
\[C{{H}_{3}}CHO\]
done
clear
C)
\[{{C}_{6}}{{H}_{5}}CHO\]
done
clear
D)
\[C{{H}_{3}}COC{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 93) The solution obtained by adding water to alcohol, shows:
A)
+ve deviation from Raoult's law
done
clear
B)
ideal behavior
done
clear
C)
-ve deviation from Raoult's law
done
clear
D)
application of Henry's law
done
clear
View Answer play_arrow
question_answer 94) Hinsberg's reagent is ;
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 95) According to Bohr, the angular momentum of an electron, in any orbit, should be:
A)
\[h/2\pi \]
done
clear
B)
\[nh/2\pi \]
done
clear
C)
\[h/m\upsilon \]
done
clear
D)
\[2\pi /nh\]
done
clear
View Answer play_arrow
question_answer 96) \[C{{H}_{3}}-C{{H}_{2}}-Cl\xrightarrow{ale.\text{ }KOH}A,\]the product is:
A)
\[C{{H}_{3}}C{{H}_{2}}OK\]
done
clear
B)
\[C{{H}_{3}}CHO\]
done
clear
C)
\[C{{H}_{3}}C{{H}_{2}}OC{{H}_{2}}C{{H}_{3}}\]
done
clear
D)
\[C{{H}_{2}}=C{{H}_{2}}\]
done
clear
View Answer play_arrow
question_answer 97) According to Hess's law, the heat of reaction depends upon:
A)
initial condition of reactants
done
clear
B)
initial and final conditions of reactants
done
clear
C)
intermediate path of the reaction
done
clear
D)
end conditions of reactants
done
clear
View Answer play_arrow
question_answer 98) Which of the following reaction is a redox reaction?
A)
\[{{P}_{2}}{{O}_{5}}+2{{H}_{2}}O\xrightarrow{{}}{{H}_{4}}{{P}_{2}}{{O}_{7}}\]
done
clear
B)
\[2AgN{{O}_{3}}+BaC{{l}_{2}}\to 2AgCl+Ba{{(N{{O}_{3}})}_{2}}\]
done
clear
C)
\[BaC{{l}_{2}}+{{H}_{2}}S{{O}_{4}}\xrightarrow{{}}BaS{{O}_{4}}+2HCl\]
done
clear
D)
\[Cu+2AgN{{O}_{3}}\xrightarrow{{}}2Ag+Cu{{(N{{O}_{3}})}_{2}}\]
done
clear
View Answer play_arrow
question_answer 99) pH of a solution of 10 mL 1N sodium acetate and 50 mL 2N acetic acid\[({{K}_{a}}=1.8\times {{10}^{-5}}),\]is approximately:
A)
4
done
clear
B)
5
done
clear
C)
6
done
clear
D)
7
done
clear
View Answer play_arrow
question_answer 100) What is 'A' in the following reaction? \[2F{{e}^{3+}}(aq)+S{{n}^{2+}}(aq)\xrightarrow{{}}2F{{e}^{2+}}(aq)+A\]
A)
\[S{{n}^{3+}}(aq)\]
done
clear
B)
\[S{{n}^{4}}+(aq)\]
done
clear
C)
\[S{{n}^{2+}}(aq)\]
done
clear
D)
\[Sn\]
done
clear
View Answer play_arrow
question_answer 101) The pH is less than 7, of the solution of:
A)
\[FeC{{l}_{3}}\]
done
clear
B)
\[NaCN\]
done
clear
C)
\[NaOH\]
done
clear
D)
\[NaCl\]
done
clear
View Answer play_arrow
question_answer 102) A reaction is catalysed by 'X'. Here 'X' :
A)
decreases the rate constant of reaction
done
clear
B)
does not affect the equilibrium constant of reaction
done
clear
C)
decreases the enthalpy of reaction
done
clear
D)
decreases the activation energy
done
clear
View Answer play_arrow
question_answer 103) For a reaction,\[A+B\xrightarrow{{}}\]product, it was found that rate of reaction increases four times if concentration of 'A' is doubled, but the rate of reaction remains unaffected if concentration of 'B' is doubled. Hence, the rate law for reaction is:
A)
\[rate=k[A][B]\]
done
clear
B)
\[rate=k{{[A]}^{2}}\]
done
clear
C)
\[rate=k{{[A]}^{2}}{{[B]}^{1}}\]
done
clear
D)
\[rate=k{{[A]}^{2}}{{[B]}^{2}}\]
done
clear
View Answer play_arrow
question_answer 104) The following four solutions are kept in separate beakers and copper metal is put in each of them. Which solution will become blue after some time?
A)
\[AgN{{O}_{3}}\]solution
done
clear
B)
\[Zn{{(N{{O}_{3}})}_{2}}\]solution
done
clear
C)
\[Ba{{(N{{O}_{3}})}_{2}}\]solution
done
clear
D)
\[NaN{{O}_{3}}\]solution
done
clear
View Answer play_arrow
question_answer 105) The\[E{}^\circ \]for half cells\[Fe/F{{e}^{2+}}\]and\[Cu/C{{u}^{2+}}\] are\[-0.44\text{ }V\]and\[+0.32\text{ }V\]respectively. Then:
A)
\[C{{u}^{2+}}\]oxidises\[Fe\]
done
clear
B)
\[C{{u}^{2+}}\]oxidises \[F{{e}^{2+}}\]
done
clear
C)
\[Cu\]oxidises\[F{{e}^{2+}}\]
done
clear
D)
\[Cu\]reduces\[F{{e}^{2+}}\]
done
clear
View Answer play_arrow
question_answer 106) The values of\[{{K}_{sp}}\]for\[CuS,A{{g}_{2}}S\]and\[HgS\]are \[{{10}^{-31}},~{{10}^{42}}\]and\[{{10}^{-54}}\]respectively. The correct order of their solubility in water is:
A)
\[A{{g}_{2}}S>HgS>CuS\]
done
clear
B)
\[HgS>CuS>A{{g}_{2}}S\]
done
clear
C)
\[HgS>A{{g}_{2}}S>CuS\]
done
clear
D)
\[A{{g}_{2}}S>CuS>HgS\]
done
clear
View Answer play_arrow
question_answer 107) Phenolphthalein is not suitable for the titration of:
A)
\[NaOH\]vs \[{{(COOH)}_{2}}\]
done
clear
B)
\[KOH\]vs\[{{H}_{2}}S{{O}_{4}}\]
done
clear
C)
\[{{K}_{2}}C{{O}_{3}}\]vs\[HCl\]
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 108) For a gaseous reaction,\[{{C}_{2}}{{H}_{4}}+{{H}_{2}}{{C}_{6}}{{H}_{6}};\] \[\Delta H=-32.7\text{ }kcal.\] The concentration of\[{{C}_{2}}{{H}_{4}}\]can be decreased by:
A)
increasing pressure
done
clear
B)
increasing amount of\[{{H}_{2}}\]
done
clear
C)
decreasing temperature
done
clear
D)
all of these
done
clear
View Answer play_arrow
question_answer 109) \[PC{{l}_{5}}\]was heated in a\[10\text{ }d{{m}^{3}}\]container at \[250{}^\circ C\]\[PC{{l}_{5}}(g)PC{{l}_{3}}(g)+C{{l}_{2}}(g)\]0.1 mol\[PC{{l}_{5}}\] and 0.2 mol\[C{{l}_{2}}\]was found at equilibrium. The equilibrium constant for the reaction is:
A)
0.025
done
clear
B)
0.04
done
clear
C)
0.02
done
clear
D)
0.05
done
clear
View Answer play_arrow
question_answer 110) In the reaction, \[{{H}_{2}}S{{O}_{4}}+{{H}_{2}}O{{H}_{3}}{{O}^{+}}+HSO_{4}^{-},\] the conjugate acid of water is:
A)
\[{{H}_{3}}{{O}^{+}}\]
done
clear
B)
\[{{H}_{2}}O\]
done
clear
C)
\[HSO_{4}^{-}\]
done
clear
D)
\[SO_{4}^{2-}\]
done
clear
View Answer play_arrow
question_answer 111) The iron, obtained from blast furnace is:
A)
pig iron
done
clear
B)
cast iron
done
clear
C)
wrought iron
done
clear
D)
steel
done
clear
View Answer play_arrow
question_answer 112) The molecular formula of 'salt petre' is:
A)
\[KN{{O}_{3}}\]
done
clear
B)
\[NaN{{O}_{3}}\]
done
clear
C)
\[NaCl\]
done
clear
D)
\[N{{a}_{2}}S{{O}_{4}}\]
done
clear
View Answer play_arrow
question_answer 113) Transition metals are related to which block?
A)
's' block
done
clear
B)
'p' block
done
clear
C)
'd' block
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 114) 36 g water and 828 g ethyl alcohol form an ideal solution. The mole fraction of water in it, is:
A)
1.0
done
clear
B)
0.7
done
clear
C)
0.4
done
clear
D)
0.1
done
clear
View Answer play_arrow
question_answer 115) The general electronic configuration of transition elements, is:
A)
\[(n-1){{d}^{1-5}}n{{s}^{0}}\]
done
clear
B)
\[(n-1){{d}^{1-10}}n{{s}^{1}}\]
done
clear
C)
\[(n-1){{d}^{1-10}}n{{s}^{2}}\]
done
clear
D)
\[(n-1){{d}^{1-10}}n{{s}^{1-2}}\]
done
clear
View Answer play_arrow
question_answer 116) Which one of these, is not a colligative property?
A)
vapour pressure
done
clear
B)
osmotic pressure
done
clear
C)
lowering in freezing point
done
clear
D)
Elevation in boiling point
done
clear
View Answer play_arrow
question_answer 117) Which of the following element does not show variable valency?
A)
Ni
done
clear
B)
Zn
done
clear
C)
Cu
done
clear
D)
Mn
done
clear
View Answer play_arrow
question_answer 118) Hydrogen in respect to chlorine, is:
A)
electropositive
done
clear
B)
electronegative
done
clear
C)
neutral
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 119) The osmotic pressure of which solution is maximum? (consider that decimormal solution of each is 90% dissociated):
A)
Aluminium sulphate
done
clear
B)
Barium chloride
done
clear
C)
Sodium sulphate
done
clear
D)
A mixture of equal volumes of and
done
clear
View Answer play_arrow
question_answer 120) Which of the following elements, is most electronegative?
A)
F
done
clear
B)
O
done
clear
C)
Ne
done
clear
D)
Na
done
clear
View Answer play_arrow
question_answer 121) All worms are :
A)
triploblastic
done
clear
B)
segmented
done
clear
C)
endo-parasites
done
clear
D)
free - living
done
clear
View Answer play_arrow
question_answer 122) In humans schizont stage of Plasmodium is found in side:
A)
liver cells only
done
clear
B)
liver, spleen and blood cells
done
clear
C)
RBCs and liver cells
done
clear
D)
RBCs only
done
clear
View Answer play_arrow
question_answer 123) Pearl oyster is a mollusc which belongs to class :
A)
Cephalopoda
done
clear
B)
Pelecypoda
done
clear
C)
Scaphopoda
done
clear
D)
Gastropoda
done
clear
View Answer play_arrow
question_answer 124) In honey bee royal jelly is secreted from :
A)
corp gland
done
clear
B)
wax gland
done
clear
C)
pharyngeal gland
done
clear
D)
salivary gland
done
clear
View Answer play_arrow
question_answer 125) Which of the following is a coelenlerate?
A)
Sea lily
done
clear
B)
Sea squirt
done
clear
C)
Sea cucumber
done
clear
D)
Sea fan
done
clear
View Answer play_arrow
question_answer 126) Which of the following is correct pair of disease and its causing agent?
A)
Soft - sour - Bacillus breueis
done
clear
B)
Gonorrhoea - Entamoeba
done
clear
C)
Syphilis - Troponema pallidum
done
clear
D)
Urethritis - Bacillus anthracis
done
clear
View Answer play_arrow
question_answer 127) Lac is obtained from :
A)
Laccifer
done
clear
B)
Bombyx
done
clear
C)
Dactylopius
done
clear
D)
Lytta
done
clear
View Answer play_arrow
question_answer 128) As per classification which of the following is correct?
A)
Ascaris, Pherctima, Grasshopper
done
clear
B)
Hydra, Pterido, Leucosotenia
done
clear
C)
Starfish, Grasshopper, Solen
done
clear
D)
Pila, Dentalium, Octopus
done
clear
View Answer play_arrow
question_answer 129) As insecticides reach at higher trophic level in food chain their concentration :
A)
increases
done
clear
B)
become irregular
done
clear
C)
remain constant
done
clear
D)
decreases
done
clear
View Answer play_arrow
question_answer 130) Kaziranga National Park is famous for :
A)
Rhinoceros
done
clear
B)
Tiger
done
clear
C)
Asiatic lion
done
clear
D)
Nilgai
done
clear
View Answer play_arrow
question_answer 131) Which of the following cells arc associated with immune system of body?
A)
Neutrophils
done
clear
B)
Macrophages
done
clear
C)
Lymphocytes
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 132) Kala-azar disease is transmitted by :
A)
Tabinus
done
clear
B)
Solomax
done
clear
C)
Glossina
done
clear
D)
Phlebotomus
done
clear
View Answer play_arrow
question_answer 133) To obtain aminoacids and other organic compounds, Stanley Miller and Urey used in his experiment :
A)
mixture of \[{{O}_{2}},{{N}_{2}},C{{H}_{4}}\]and\[{{H}_{2}}O\]
done
clear
B)
mixture of \[N{{H}_{3}},{{H}_{2}},{{N}_{2}}\]and \[{{H}_{2}}O\]
done
clear
C)
mixture of \[{{H}_{2}},N{{H}_{3}},C{{H}_{4}},\] and \[{{H}_{2}}O\]
done
clear
D)
mixture of \[{{H}_{2}},{{O}_{2}},C{{O}_{2}}\]and \[{{H}_{2}}O\]
done
clear
View Answer play_arrow
question_answer 134) Commercial silk is obtained from :
A)
cocoon/pupa
done
clear
B)
caterpillar
done
clear
C)
adult moth
done
clear
D)
both egg and adult moth
done
clear
View Answer play_arrow
question_answer 135) Interbreeding population of animals is called :
A)
sub-species
done
clear
B)
species
done
clear
C)
community
done
clear
D)
genus
done
clear
View Answer play_arrow
question_answer 136) Which of the following is a living fossil?
A)
Latimeria
done
clear
B)
Amia
done
clear
C)
Hippocampus
done
clear
D)
Exocoetus
done
clear
View Answer play_arrow
question_answer 137) Continuous bleeding from an injured part of body is due to deficiency of :
A)
vitamin A
done
clear
B)
vitamin B
done
clear
C)
vitamin K
done
clear
D)
vitamin E
done
clear
View Answer play_arrow
question_answer 138) In which of the following class of Annelida, one pair ovaries and several pair testes are found ?
A)
Archiannelida
done
clear
B)
Hirudinea
done
clear
C)
Oligochaeta
done
clear
D)
Polychaeta
done
clear
View Answer play_arrow
question_answer 139) Sex determination in humans takes place by:
A)
sex chromosomes of father
done
clear
B)
measurement of sperm
done
clear
C)
measurement of ovum
done
clear
D)
sex chromosomes of mother
done
clear
View Answer play_arrow
question_answer 140) The term genetic engineering is used for:
A)
blotting technique
done
clear
B)
RNA reaction technique
done
clear
C)
protien synthesis technique
done
clear
D)
recombinant DNA technique
done
clear
View Answer play_arrow
question_answer 141) Turner's syndrome chromosome pattern is:
A)
XO
done
clear
B)
XXX
done
clear
C)
XYY
done
clear
D)
XXY
done
clear
View Answer play_arrow
question_answer 142) The main cause of Edward syndrome Patau syndrome and Down syndrome is:
A)
mutation of gene
done
clear
B)
change in both autosomes and heterosomes
done
clear
C)
change in autosomes
done
clear
D)
change in heterosomes
done
clear
View Answer play_arrow
question_answer 143) The number of chromosomes in Klinefelter's syndrome are:
A)
47
done
clear
B)
46
done
clear
C)
45
done
clear
D)
44
done
clear
View Answer play_arrow
question_answer 144) The eggs of eutherian mammals are :
A)
mesolecithal type
done
clear
B)
microlecithal type
done
clear
C)
telolecithal type
done
clear
D)
megalecithal type
done
clear
View Answer play_arrow
question_answer 145) Which of the following steroid se hormone influenced secondary se organs ?
A)
Progesterone
done
clear
B)
Oestrogen
done
clear
C)
LH
done
clear
D)
LTH
done
clear
View Answer play_arrow
question_answer 146) The genotype of a man having female sexual characters will be :
A)
XXY
done
clear
B)
XYY
done
clear
C)
XO
done
clear
D)
XXX
done
clear
View Answer play_arrow
question_answer 147) The universal recipient blood group is :
A)
O (+ ve)
done
clear
B)
AB ((+ve)
done
clear
C)
O (-ve)
done
clear
D)
AB (-ve)
done
clear
View Answer play_arrow
question_answer 148) Which of the following is a X-Iinked disease?
A)
Tetany
done
clear
B)
Colorblindness
done
clear
C)
Xeroderma
done
clear
D)
Hypertricosis
done
clear
View Answer play_arrow
question_answer 149) The condition in which there are more than two complete set of chromosome is called :
A)
polytene
done
clear
B)
monoploidy
done
clear
C)
polyploidy
done
clear
D)
aneuploidy
done
clear
View Answer play_arrow
question_answer 150) In human the inheritance of sex linkage takes place through :
A)
autosomes
done
clear
B)
Y - chromosome
done
clear
C)
X - chromosome
done
clear
D)
both 'b' and 'c'
done
clear
View Answer play_arrow
question_answer 151) Hybridoma technology is used to :
A)
kill the cancer cells
done
clear
B)
formation of somaclonal antibodies
done
clear
C)
formation of somatic hybrids
done
clear
D)
formation of antibiotics
done
clear
View Answer play_arrow
question_answer 152) Hydrophytes are characterised by :
A)
thick and large leaf
done
clear
B)
delicate and mucilagenous stem
done
clear
C)
short spinous stem
done
clear
D)
all of the above
done
clear
View Answer play_arrow
question_answer 153) Yeast is used in the production of :
A)
acetic acid
done
clear
B)
citric acid
done
clear
C)
ethyl alcohol
done
clear
D)
curd
done
clear
View Answer play_arrow
question_answer 154) The vaccine of Hepatitis-B is a :
A)
first generation vaccine
done
clear
B)
interferon
done
clear
C)
second generation vaccine
done
clear
D)
third generation vaccine
done
clear
View Answer play_arrow
question_answer 155) The term 'ecosystem' was coined by :
A)
E.P. Odum
done
clear
B)
A.G. Tansley
done
clear
C)
Carl Mobious
done
clear
D)
W. Clementus
done
clear
View Answer play_arrow
question_answer 156) Streptomycin was isolated first time by :
A)
Hemming
done
clear
B)
Robert Koch
done
clear
C)
N. Borlaug
done
clear
D)
S. Waksman
done
clear
View Answer play_arrow
question_answer 157) Which of the following micro-organism is used for conversion of milk into curd ?
A)
Xanthomonas citri
done
clear
B)
Bacillus megatherium
done
clear
C)
Acefobacter aceti
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 158) Cotton belongs to family :
A)
Solanaceae
done
clear
B)
Cucurbitaceae
done
clear
C)
Leguminoseae
done
clear
D)
Malvaceae
done
clear
View Answer play_arrow
question_answer 159) Terpentine is obtained from :
A)
flowers of angiosperms
done
clear
B)
wood of gymnosperms
done
clear
C)
pteridophytes
done
clear
D)
bacteria
done
clear
View Answer play_arrow
question_answer 160) Kohler and Milstein developed biotechnology for :
A)
monoclonal antibodies
done
clear
B)
steroid synthesis
done
clear
C)
immobilization of enzymes
done
clear
D)
myeloma
done
clear
View Answer play_arrow
question_answer 161) Which of the following is formed by the reactions of UV-rays of sunlight?
A)
\[S{{O}_{2}}\]
done
clear
B)
CO
done
clear
C)
Ozone
done
clear
D)
Florides
done
clear
View Answer play_arrow
question_answer 162) In India highest amount of coal is present in :
A)
West Bengal
done
clear
B)
Maharastra
done
clear
C)
Jharkhand
done
clear
D)
Assam,
done
clear
View Answer play_arrow
question_answer 163) Genetically engineered human insulin is called :
A)
humulin
done
clear
B)
haematin
done
clear
C)
hybridoma
done
clear
D)
hybrid
done
clear
View Answer play_arrow
question_answer 164) The food chain starts from :
A)
consumer
done
clear
B)
producer
done
clear
C)
decomposer
done
clear
D)
nitrogen fixation
done
clear
View Answer play_arrow
question_answer 165) Humulin is a :
A)
form of chitin
done
clear
B)
human insulin
done
clear
C)
digestive enzyme
done
clear
D)
antibiotics
done
clear
View Answer play_arrow
question_answer 166) The substrate for photorespiration is :
A)
glycolate
done
clear
B)
acetyl Co-A
done
clear
C)
pyruvic acid
done
clear
D)
glucose
done
clear
View Answer play_arrow
question_answer 167) Pinus seed is originated in :
A)
capsule
done
clear
B)
microsporophyll
done
clear
C)
microsporangia
done
clear
D)
megasporophyll
done
clear
View Answer play_arrow
question_answer 168) The endosperm of Pinus is :
A)
haploid
done
clear
B)
diploid
done
clear
C)
triploid
done
clear
D)
polyploidy
done
clear
View Answer play_arrow
question_answer 169) Which of the following is the product of glucose fermentation by yeast?
A)
\[{{C}_{6}}{{H}_{12}}{{O}_{6}}\]
done
clear
B)
\[{{C}_{2}}{{H}_{5}}OH\]
done
clear
C)
\[({{C}_{6}}{{H}_{10}}{{O}_{5}})\]
done
clear
D)
\[C{{H}_{2}}OH\]
done
clear
View Answer play_arrow
question_answer 170) From which part of Cocos nucifera, coir is obtained :
A)
Endocarp
done
clear
B)
Mesocarp
done
clear
C)
Epicarp
done
clear
D)
Any part of fruit
done
clear
View Answer play_arrow
question_answer 171) The formation of embryo without fusion of gametes is termed as :
A)
apospory
done
clear
B)
isogamy
done
clear
C)
apogamy
done
clear
D)
syngamy
done
clear
View Answer play_arrow
question_answer 172) The pesticide used as preventive measure in buildings is :
A)
aldrin
done
clear
B)
dieldrin
done
clear
C)
endrin
done
clear
D)
DDT
done
clear
View Answer play_arrow
question_answer 173) Which of the following is not a pollutant?
A)
Hydrogen
done
clear
B)
Carbon dioxide
done
clear
C)
Sulphur dioxide
done
clear
D)
Carbon monoxide
done
clear
View Answer play_arrow
question_answer 174) Main air pollutant is :
A)
\[CO\]
done
clear
B)
\[C{{O}_{2}}\]
done
clear
C)
\[S\]
done
clear
D)
\[{{N}_{2}}\]
done
clear
View Answer play_arrow
question_answer 175) Cheese and yogurt are the products of:
A)
dehydration
done
clear
B)
fermentation
done
clear
C)
pasteurization
done
clear
D)
distillation
done
clear
View Answer play_arrow
question_answer 176) Which of the following maintain the constant number of chromosomes generation after generation?
A)
Meiosis
done
clear
B)
Endomitosis
done
clear
C)
Mitosis
done
clear
D)
Amitosis
done
clear
View Answer play_arrow
question_answer 177) Plant cell is differ from animal cell because of:
A)
the presence of cell wall and absence of chlorophyll in plant cell
done
clear
B)
the presence of cell wall and chlorophyll in plant cell
done
clear
C)
the absence of cell wall and presence of chloroplast in animal cell
done
clear
D)
the absence of cell wall and presence of chlorophyll in plant cell
done
clear
View Answer play_arrow
question_answer 178) DNA remains absent in :
A)
chloroplast
done
clear
B)
nucleus
done
clear
C)
peroxysomes
done
clear
D)
chromosome
done
clear
View Answer play_arrow
question_answer 179) Photosynthetic unit is :
A)
glyoxysome
done
clear
B)
spherosome
done
clear
C)
microsome
done
clear
D)
quantasome
done
clear
View Answer play_arrow
question_answer 180) The ?power house' of cell is :
A)
nucleus
done
clear
B)
golgi body
done
clear
C)
mitochondria
done
clear
D)
chloroplast
done
clear
View Answer play_arrow