A) + 5
B) +3
C) + 6
D) - 10
Correct Answer: C
Solution :
| When \[{{H}_{2}}{{O}_{2}}\] is added to an acidified solution of a dichromate, \[C{{r}_{2}}O_{7}^{2-}\], a deep blue coloured complex, chromic peroxide, \[Cr{{O}_{5}}\] \[[or\,CrO\,{{({{O}_{2}})}_{2}}]\] is formed. |
| \[C{{r}_{2}}O_{7}^{2-}+2{{H}^{+}}+4{{H}_{2}}{{O}_{2}}\xrightarrow{\,}\underset{Chromic\,peroxide}{\mathop{2CrO{{({{O}_{2}})}_{2}}}}\,+5{{H}_{2}}O\] |
| This deep blue coloured complex has the following structure |
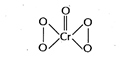 |
| Oxidation state of Cr is +6 due to the presence of two peroxide linkages, which can be calculated as |
| Cr peroxide normal |
| \[x+(-1)4+(-2)=0\] |
| \[x-6=0\] |
| \[x=+6\] |
You need to login to perform this action.
You will be redirected in
3 sec
