Answer:
(i)
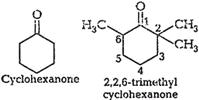 In 2,2,6 trimethyl cyclohexanone, three
methyl groups arepresents at a-position with respect to the ketonic
In 2,2,6 trimethyl cyclohexanone, three
methyl groups arepresents at a-position with respect to the ketonic ![]() group.
Therefore, these groups cause
steric hindrance during thenucleophilic attack of
group.
Therefore, these groups cause
steric hindrance during thenucleophilic attack of ![]() ion so
cyanohydrin is not formed. Dueto the absence of methyl groups in cyclohexanone,
there is nosteric hindrance and cyanohydrin is formed.
(ii) Resonance in
semicarbazide
ion so
cyanohydrin is not formed. Dueto the absence of methyl groups in cyclohexanone,
there is nosteric hindrance and cyanohydrin is formed.
(ii) Resonance in
semicarbazide
![]() Semicarbazide has two amino
Semicarbazide has two amino ![]() groups,
out of which one isinvolved in resonance as shown above. Electron-density on
this
groups,
out of which one isinvolved in resonance as shown above. Electron-density on
this![]() decreases
and it does not act as a nucleophile.
But the other
decreases
and it does not act as a nucleophile.
But the other ![]() group
(attached to NH) has lone pair ofelectrons which, are not involved in
resonance. So, this pair isavailable for the nucleophilic attack on carbonyl
group
group
(attached to NH) has lone pair ofelectrons which, are not involved in
resonance. So, this pair isavailable for the nucleophilic attack on carbonyl
group ![]() of aldehydes and/or
ketones.
(iii) Esterification is a reversible
reaction.
of aldehydes and/or
ketones.
(iii) Esterification is a reversible
reaction.
![]() When the sufficient amount of products
is formed, the rate offorward reaction decreases and the reverse reaction
begins. Toavoid this condition i.e., in order to shift the equilibrium in
forwarddirection, the concentration of products (ester and/or water)should be
decreased. (Le-Chatelier's principle). So, water shouldbe removed from time to
time.
When the sufficient amount of products
is formed, the rate offorward reaction decreases and the reverse reaction
begins. Toavoid this condition i.e., in order to shift the equilibrium in
forwarddirection, the concentration of products (ester and/or water)should be
decreased. (Le-Chatelier's principle). So, water shouldbe removed from time to
time.
You need to login to perform this action.
You will be redirected in
3 sec
