Answer:
Step I: To determine the molecular formula
of thecompound.
Empirical formula of the given
organic compound
Element
Percentage
Atomic mass
No. of moles
Simplest molar ratio
C
H
O
69.77
11.63
(100-69.77-11.63)
= 18.60
12
1
16
![]()
![]()
![]()
![]()
![]()
![]()
![]() .
Molecular formula = n x
(empirical formula)
where,
.
Molecular formula = n x
(empirical formula)
where, ![]() Given, molecular mass = 86
Empirical formula mass of
Given, molecular mass = 86
Empirical formula mass of ![]()
![]()
![]()
![]() Molecular formula
Molecular formula ![]() Step II: Predicting the
structure of the compound.
Criteria:
1. Formation of addition
compound with
Step II: Predicting the
structure of the compound.
Criteria:
1. Formation of addition
compound with ![]() depictsthe
presence of aldehyde or ketone.
2. The given compound does not
reduce Tollen's reagent but givespositive iodoform test, so it is a methyl
ketone.
3. On oxidation, the compound
forms a mixture of ethanoic andpropanoic acid, so it is
depictsthe
presence of aldehyde or ketone.
2. The given compound does not
reduce Tollen's reagent but givespositive iodoform test, so it is a methyl
ketone.
3. On oxidation, the compound
forms a mixture of ethanoic andpropanoic acid, so it is
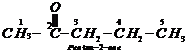 Step III: Chemical equations:
1.
Step III: Chemical equations:
1. 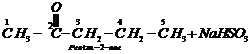
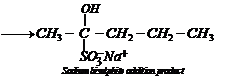 2.
2. 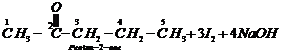
![]()
![]()
![]() 3.
3. 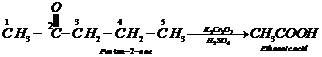
You need to login to perform this action.
You will be redirected in
3 sec
