Answer:
(i)
Since compound 'B' gives Fehling's test, therefore, it must be aldehyde.
Further since aldehyde 'B' gives iodoform on treatment with ![]() and NaOH, therefore, 'B'
must be acetaldehyde
and NaOH, therefore, 'B'
must be acetaldehyde ![]() .
(ii) Since alkene 'A' (MP
.
(ii) Since alkene 'A' (MP![]() ) contains five carbon
atoms and one of the products of ozonolysis is ?B?
) contains five carbon
atoms and one of the products of ozonolysis is ?B? ![]() which contains two carbon
atoms, therefore, the other product of ozonolys is, i.e., 'C' must contain three
carbon atoms,
(iii) Since compound 'C' does not give Fehling's test, it must be
a ketone. Further since ketone 'C' contain three carbon atoms and gives
iodoform on treatment with
which contains two carbon
atoms, therefore, the other product of ozonolys is, i.e., 'C' must contain three
carbon atoms,
(iii) Since compound 'C' does not give Fehling's test, it must be
a ketone. Further since ketone 'C' contain three carbon atoms and gives
iodoform on treatment with ![]() and
NaOH, therefore, ketone 'C' must be acetone
(iv) Write the products of ozonolysis, i.e., 'B'
and
NaOH, therefore, ketone 'C' must be acetone
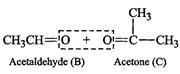
(iv) Write the products of ozonolysis, i.e., 'B' ![]() and 'C'
and 'C' ![]() side by side with their
C = O groups facing each other. Remove the oxygen atoms and join the remaining
fragments by a doubles bond, the structure of alkene 'A' is
2-methylbut-2-ene.
side by side with their
C = O groups facing each other. Remove the oxygen atoms and join the remaining
fragments by a doubles bond, the structure of alkene 'A' is
2-methylbut-2-ene.
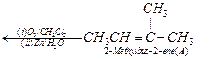 (v) Formation of iodoform from 'B' and 'C' may be
explained as follows :
(v) Formation of iodoform from 'B' and 'C' may be
explained as follows :
![]()
![]()
![]()
![]()
You need to login to perform this action.
You will be redirected in
3 sec
