Answer:
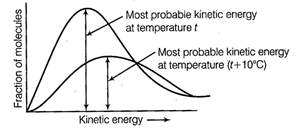
Kinetic energy is directly proportional to
the absolute temperature and the number of molecules possessing higher energies
increases with increase in temperature, i.e., most probable kinetic energy
increases with increase in temperature.
Energy of activation is related to
temperature by the following Arrhenius equation
![]() Thus, it also shows an increase
with rise in temperature.
Thus, it also shows an increase
with rise in temperature.
You need to login to perform this action.
You will be redirected in
3 sec
