Answer:
Sol.
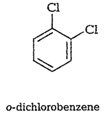
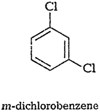
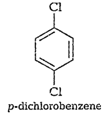 Out of the above three isomers of
dichlorobenzene, the p-isomer ismore symmetrical than other two isomers. So, it
has more closelypacked arrangement of molecules in its crystal lattice.
So,p-dichlorobenzene has a higher melting point and lower solubility ascompared
to ortho and meta isomers.
Out of the above three isomers of
dichlorobenzene, the p-isomer ismore symmetrical than other two isomers. So, it
has more closelypacked arrangement of molecules in its crystal lattice.
So,p-dichlorobenzene has a higher melting point and lower solubility ascompared
to ortho and meta isomers.
You need to login to perform this action.
You will be redirected in
3 sec
