| Column 1 | Column 11 | |
| A. B. C. D. | Simple vacancy defect Simple interstitial defect Frenkel defect Schottky defect | 1. Shown by non-ionic solids and increases density of the solid 2. Shown by ionic solids and decreases density of the solid 3. Shown by non-ionic solids and density of the solid decreases 4. Shown by ionic solids and density of the solid remains the same |
Answer:
A. ® (3) B.® W C.® (4) D. ®(2)
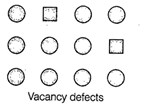
A. When some of lattice sites are vacant
in any non-ionic solid, the crystal is said to have vacancy defect and due to
decrease in number of particles present in crystal lattice the density of
crystal decreases.
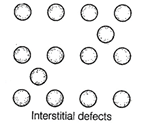
 B. Simple interstitial defect
are shown by non- ionic solids in which constituent particles is displaced from
its normal site to an interstitial site. Hence, density of solid increases.
B. Simple interstitial defect
are shown by non- ionic solids in which constituent particles is displaced from
its normal site to an interstitial site. Hence, density of solid increases.
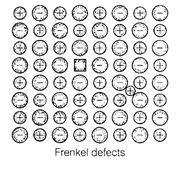
 C. Frenkel defect is shown by
ionic solids in which smaller ions get dislocated from its normal site to its
interstitial site which lead to decrease its density.
C. Frenkel defect is shown by
ionic solids in which smaller ions get dislocated from its normal site to its
interstitial site which lead to decrease its density.
 D. Schottky defect is shown by
ionic solids in which equal number of cation and anion get missed from ionic
solids and thus, density of solid decreases.
D. Schottky defect is shown by
ionic solids in which equal number of cation and anion get missed from ionic
solids and thus, density of solid decreases.

You need to login to perform this action.
You will be redirected in
3 sec
