Answer:
When molten NaCl
(Na+ is monovalent) containing a little amount of SrCl2
(Sr2+ is divalent) as impurity is crystallised, some of the sites of
Na+ ions are occupied by Sr2+. Each Sr2+
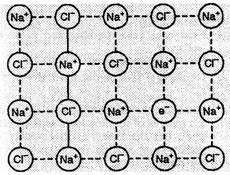 replaces two Na+
ions. One site is occupied by Sr2+ and the other site remains
vacant. The cationic vacancies thus produced are equal to the number of Sr2+
ions.
replaces two Na+
ions. One site is occupied by Sr2+ and the other site remains
vacant. The cationic vacancies thus produced are equal to the number of Sr2+
ions.
You need to login to perform this action.
You will be redirected in
3 sec
