Answer:
Alkali halides like
NaCI and KCI show this type of defect. When crystals of NaCI are heated in an
atmosphere of sodium vapour, the sodium
 atoms are deposited
on the surface of the crystal. The
atoms are deposited
on the surface of the crystal. The ![]() ions diffuse to
the surface of the crystal and combine with Na atoms to give
ions diffuse to
the surface of the crystal and combine with Na atoms to give ![]() .
This happens by loss of electron by sodium atoms to form
.
This happens by loss of electron by sodium atoms to form ![]() ions.
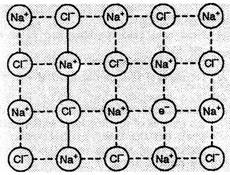
The released electrons diffuse into the crystal and occupy anionic sites
(Fig.). As a result, the crystal now has an excess of sodium. Thus, it is metal
excess defect. The anionic sites occupied by unpaired electrons are called F-centres
(from the German word Farbenzenter for colour centre). They impart yellow
colour to the crystals of
ions.
The released electrons diffuse into the crystal and occupy anionic sites
(Fig.). As a result, the crystal now has an excess of sodium. Thus, it is metal
excess defect. The anionic sites occupied by unpaired electrons are called F-centres
(from the German word Farbenzenter for colour centre). They impart yellow
colour to the crystals of ![]() . The colour
results by excitation of these electrons when they absorb energy from the
visible light falling on the crystals. Similarly, excess of lithium makes
. The colour
results by excitation of these electrons when they absorb energy from the
visible light falling on the crystals. Similarly, excess of lithium makes ![]() crystals
pink and excess of potassium makes
crystals
pink and excess of potassium makes ![]() crystals violet
(or lilac).
crystals violet
(or lilac).
You need to login to perform this action.
You will be redirected in
3 sec
