LEARNING OBJECTIVES
This lesson will help you to:—
- Understand the concept of matter and its three states.
- Learn about the physical and chemical changes in matter.
- Study the phenomenon of melting and boiling points.
- Study the phenomenon of freezing and condensation.
QULCK CONCEPT REVIEW
WATTER
- Matter is anything that has mass and occupies space. A small button takes up less space than an elephant, but they are both matter.
- Matter is made up of small particles called as atoms and molecules.
- The amount of space occupied by an object is called volume. Some objects have more volume than others.
For example, a globe occupies more space than a ruler does. The globe has more volume than the ruler.
- Mass is the measure of how much material makes up the object. Everything that has volume and has mass is matter.
- Property of Matter: Property is something about an object that can be observed, such as its size, shape, colour, smell, or sound. All matter has property.
- There are three forms of the states of matter.
Real-Life Example
- We all have seen small white coloured naphthalene balls. These are put in the bathrooms as fresheners and in bags to protect the clothes from moths. These naphthalene balls are an example of sublimation, where these solid balls directly get converted to gas without the intermediate liquid state.
They are solids, liquids, and gases.
- Solid has a definite shape and volume.
- Liquid has a definite volume but no definite shape.
- Gas has no definite shape or volume.
- Particles are different in different states of matter. The particles in liquids are much farther apart than particles in solids. Particles in solids pull towards each other. A solid can keep its shape because its particles stay close together. Particles in solids can move back and forth but they can't change places with another particle. Particles in liquids are farther apart. The pull between particles in liquids is weaker than the pull in solids. Liquids can change shapes because the particles can move around each other and change places. Particles in gases do not pull together strongly. Gas particles can move around more than the particles in liquids and solids. A gas can move to fill any space because its particles move freely.
- Everything in our world has matter in one of these three forms.
- Matter can change in different ways. There are physical changes and chemical changes. Some matter can change states. That means some matter can change from a solid to a liquid, or from a liquid to a gas.
- Matter changes state when more energy is added to it. Energy is often added in the form of heat or pressure.
- If you change something physically, it may look, act, or feel different, but it is still the same thing. The molecules haven't changed.
For example: if you tear a piece of paper it looks different, but it is still a piece of paper. That is a physical change.
- An ice cube, a solid, becomes a liquid when it melts. You can take the liquid and turn it back into a solid by freezing it. The change is reversible. It is a different state, but it is still water. This is called a PHYSICAL CHANGE. The state has changed, but it is still the same thing - water.
- Changing physical states of matter is about changing densities, pressures, temperatures, and other physical properties. The basic chemical structure does not change when there is a physical change.
Misconcept/concept
Misconcept: Condensation is when air turns into a liquid.
Concept: Condensation is water vapour in the air that cools enough to become a liquid.
Misconcept: Condensation on the outside of a container is water that seeped (or sweated) through the walls of the container.
Concept: Condensation of water vapour happens when the water vapour in air comes in contact with a cool surface.
Misconcept: Steam is hot air
Concept: Steam is water vapour.
Amazing Facts
- When rocks get really hot they turn into a liquid called magma or lava.
- We use natural gas in our homes in its gas state, but when it’s shipped in ocean tankers it’s shipped in a liquid state to save on space.
- When liquid gasoline is burned in a car, it turns into various gases which go into the air from the exhaust pipe.
- A fourth state of matter also exists, called as plasma. Plasma occurs at very high temperatures and can be found in stars and lightning bolts.
CHEMICAL CHANGE'
- Matter con also change and become something completely different that is called a CHEMICAL CHANGE. A chemical change is irreversible; that
- Means it cannot change back into original substance.
- A chemical change happens when the atoms in a molecule are moved round or when atoms are added or taken away.
- Think about baking a cake. The batter is a liquid mixture of flour, sugar, water and other ingredients. When you hear it in the oven, the batter turns into a yummy solid. You cannot change it back into flour, sugar, water and the other ingredients. The batter has undergone a chemical change hat is irreversible and it has become something new.
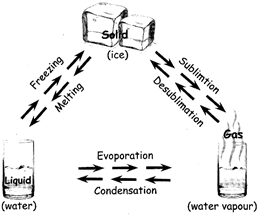
When a solid turns into a liquid it is called melting. There is a temperature at which this happens called the melting point. As the energy in the molecules increases from a rise in temperature, the molecules start moving faster. Soon they have enough energy to break free of their rigid structure and start moving around more easily. The matter becomes a liquid. The melting point for water is 0 degree C (32 degrees F). When the opposite happens and a liquid turns into a solid, it is called freezing.
BOILING AND CONDENSATION
When a liquid becomes a gas it is called boiling or vaporization. Again, at a certain temperature called the boiling point, the molecules will gain enough energy to break free and become a gas. The boiling point for water is 100 degrees C. When the opposite occurs and a gas becomes a liquid, it is called condensation.
EVAPORATION
- Evaporation is liquid becoming a gas that happens only on the surface of a liquid.
- Evaporation doesn't always need a high temperature to occur.
- Even though the overall energy and temperature of a liquid may be low, the molecules on the surface that are in contact with the air and gases around them, can possess high energy. These molecules on the surface will slowly become gases through evaporation.
- You can see evaporation when water on your skin dries or a puddle in the street slowly goes away.
SUBLIMATION
- Sublimation of an element or substance is a conversion between the solid and the gaseous states with no liquid intermediate stage.
- Carbon dioxide is a common example of a compound that sublimes at normal pressures.
- Scientists have always know about solids, liquids and gases. Plasma was a new idea when it was noticed by William Crookes in 1879. The scientists who worked with the Bose-Einstein Condensate (BEC) received a Nobel Prize for their work in 1995. The BFC is all about molecules that are really close to each other (even closer than atoms in a solid).

