Substance and Its Nature
Introduction
Chemistry is the branch of science which deals with the composition of matter and also the
Physical and Chemical characteristics associated with the different material objects.
A French chemist, Lavoisier (1743 – 1793) is regarded as father of modem chemistry.
Substance and Its Nature
Anything that occupies space, possesses mass and can be felt by any one or more of our senses is called matter.
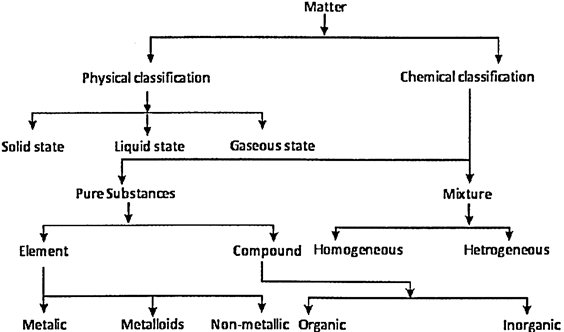
- Anything which has mass and occupies space is called matter.
- Matter can be classified as pure substances or mixtures.
- A pure substance may either contain constituent particles of only one kind or of different kinds. A pure substance has a fixed composition.
- An element is a basic form of matter which cannot be broken down into simpler substances by any physical or chemical means.
- Elements can be broadly classified as metals, non-metals and metalloids.
- Metals are one category of elements that have lustre. They conduct heat and electricity.
- They are sonorous .They are malleable and ductile.
- Non metals do not have lustre, are not sonorous and are bad conductors of heat and electricity.
- Metalloids are elements having properties intermediate between those of metals and non-metals.
- A compound is a pure substance composed of two or more elements chemically combined in a fixed proportion. It can be broken down into simpler substances by chemical or electrochemical methods.
- A mixture contains two or more elements or compounds which are mixed together in any proportion. In a mixture no new compound is formed. A mixture shows the properties of the constituent substances.
- Mixtures are classified are homogeneous or heterogeneous mixture
- Mixtures whose components mix completely with each other to make a uniform composition are called homogeneous mixtures.
- A heterogeneous mixture has a non - uniform composition.
- The ability of a substance to dissolve in another substance is called solubility.
- Homogeneous mixture of two or more substances is called a solution.
- Component of a solution present in small quantity is called a solute.
- Component of a solution present in large excess is called a solvent.
- Solution with high solute concentration is called concentrated solution and those with low concentration is called dilute solution.
- A solution that has dissolved maximum amount of solute at any particular temperature is said to be a saturated solution.
- The smallest particle of an element is called an atom. An atom can take part in chemical combination and does not occur free in nature. The atom of the hydrogen is the smallest and lightest. Eg - Na, K, Ca, H etc.
- A molecule is the smallest particle of an element or compound that can have a stable and independent existence.
\[Eg-{{O}_{2}},{{N}_{2}},C{{I}_{2}}{{P}_{4}},\] etc.
- A mole is a collection of \[6.023\times \text{1}{{0}^{3}}\]particles.
- The number \[6.023\times {{10}^{23}}\] is called Avogadro's Number,
- It is the ratio of mass of one atom of the element to 1/12th part of the mass of one atom of carbon – 12.
- It indicates how many times one molecule of a substance is heavier in comparison to
- 1/12th mass of one atom of Carbon –12.
- Fundamental particles of an atom are Electron, Proton & Neutron.
- Electron had been discovered by J.J. Thomson.
- A proton had been discovered by Goldstein. A proton was named by Rutherford.
- A neutron had been discovered by James Chadwick.
- The number of proton or electron in an atom of the element is called atomic number.
- The sum of number of protons and neutrons in an atom of the element, is called mass number. St is denoted by A.
- Isotopes having the same atomic number but different mass number Isotopes of
\[\operatorname{Carbon}-\,_{6}^{12}C,\,_{6}^{13}C,_{6}^{14}C.\]
- Isobars having the same mass number but different atomic numbers.
\[e.g.-\,_{18}^{40}Ar,\,\,_{19}^{40}K,\,\,_{20}^{40}Ca.\]
- Isotones are the atoms of different elements having the same number of neutrons, Eg.
\[\,_{6}^{14}C,\,\,_{7}^{15}N,\,\,_{8}^{16}O.\]
- Isoelectronic are atoms/molecules/ions containing the same number of electrons. Eg.
\[{{O}^{2-}},\text{ }{{F}^{-}},\text{ }Ne,\text{ }N{{a}^{+}},M{{g}^{2+}}.\]
Commonly Asked Questions
- Which one of the following does not show the properties of the constituents?
(a) Water (b) Air
(c) Sugar solution (d) All of these
(e) None of these
Answer (A)
Explanation: Water is a compound and compounds do not show the properties of its constituents.
- Which of the following properties is different for solids, liquids and gases?
(a) Movement of molecules
(b) Particle size of the substance
(c) Mass of the substance
(d) Energy exchanges
(e) None of these
Answer (A)
Explanation: Movement of molecules is different for solid, liquids and gases because position of molecules are different in them like molecules in solid have strong intermolecular force. In liquids these molecules have less intermolecular force than solid so that molecules can move. In gases molecules have least intermolecular force hence molecules move in random motion.


- Which one of the following is an example of a mixture?
(a) Sugar (b) Brass
(c) \[C{{O}_{2}}\] (d) \[N{{O}_{2}}\]
(e) None of these
Answer: (B)
Explanation: Brass is an example of mixture because mixture shows the properties of its constituents. It is a mixture of copper and zink.
- Scattering of light by colloidal particles is know as:
(a) Tyndall effect (b) Brownian movement
(c) Reflection (d) Rectilinear propagation
(e) None of these
Answer: (A)
- Amongst element X (2, 8, 6) and Y (2, 8, 8) which is more reactive and why?
(a) X because it is a metal
(b) Y because it is non - metal
(c) X because it has 6 valence electrons
(d) Y because it is gas
(e) None of these
Answer (C)
Explanation: X is more reactive because It has 6 valance electrons.
\[\operatorname{X}-K=2,\,L=8,M=6,\] \[\operatorname{Y}-K=2,L=8,\,\,M=8\]
X has 6 valence electrons so it can gain 2 electrons to complete Its octet and Y has 8 valance electrons hence its octet is cornplete.




