Chemical Properties Of Carbonyl Compounds
Category : JEE Main & Advanced
Carbonyl compounds give chemical reactions due to carbonyl group and a-hydrogens.
Chemical reactions of carbonyl compounds can be classified into following categories.
(1) Nucleophilic addition reactions
(2) Addition followed by elimination reactions
(3) Oxidation
(4) Reduction
(5) Reactions due to a-hydrogen
(6) Condensation reactions and
(7) Miscellaneous reactions
(1) Nucleophilic addition reactions
(i) Carbonyl compounds give nucleophilic addition reaction with those reagents which on dissociation give electrophile as well as nucleophile.
(ii) If nucleophile is weak then addition reaction is carried out in the presence of acid as catalyst.
(iii) Product of addition reactions can be written as follows,
\[R\underset{+\delta }{\overset{-\delta }{\mathop{\overset{\,O}{\mathop{\overset{\,||}{\mathop{-C-}}\,}}\,}}}\,R'\ +\ \overset{+\delta }{\mathop{H}}\,-\overset{-\delta }{\mathop{Nu}}\,\ \xrightarrow{\text{Addition}}\,\,\underset{\text{Adduct}}{\mathop{\underset{\,\,Nu}{\overset{\,\,\,\,OH}{\mathop{R-\underset{|}{\overset{|}{\mathop{C}}}\,-R'}}}\,}}\,\]
In addition reactions nucleophile adds on carbonyl carbon and electrophile on carbonyl oxygen to give adduct.
(iv) Relative reactivity of aldehydes and ketones : Aldehydes and ketones readily undergo nucleophilic addition reactions. However, ketones are less reactive than aldehydes. This is due to electronic and stearic effects as explained below:
(a) Inductive effect : The relative reactivities of aldehydes and ketones in nucleophilic addition reactions may be attributed to the amount of positive charge on the carbon. A greater positive charge means a higher reactivity. If the positive charge is dispersed throughout the molecule, the carbonyl compound becomes more stable and its reactivity decreases. Now, alkyl group is an electron releasing group (+I inductive effect). Therefore, electron releasing power of two alkyl groups in ketones is more than that of one alkyl group in aldehyde. As a result, the electron deficiency of carbon atom in the carbonyl group is satisfied more in ketones than in aldehydes. Therefore, the reduced positive charge on carbon in case of ketones discourages the attack of nucleophiles. Hence ketones are less reactive than aldehydes. Formaldehyde with no alkyl groups is the most reactive of the aldehydes and ketones. Thus, the order of reactivity is:
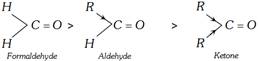
(b) Stearic effect : The size of the alkyl group is more than that of hydrogen. In aldehydes, there is one alkyl group but in ketones, there are two alkyl groups attached to the carbonyl group. The alkyl groups are larger than a hydrogen atom and these cause hindrance to the attacking group. This is called stearic hindrance. As the number and size of the alkyl groups increase, the hindrance to the attack of nucleophile also increases and the reactivity of a carbonyl decreases. The lack of hindrance in nucleophilic attack is another reason for the greater reactivity of formaldehyde. Thus, the reactivity follows the order:
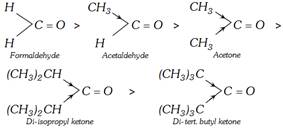
In general, aromatic aldehydes and ketones are less reactive than the corresponding aliphatic analogues. For example, benzaldehyde is less reactive than aliphatic aldehydes. This can be easily understood from the resonating structures of benzaldehyde as shown below:

It is clear from the resonating structures that due to electron releasing resonance effect of the benzene ring, the magnitude of the positive charge on the carbonyl group decreases and consequently it becomes less susceptible to the nucleophilic attack. Thus, aromatic aldehydes and ketones are less reactive than the corresponding aliphatic aldehyde and ketones. The order of reactivity of aromatic aldehydes and ketones is,
\[\underset{\text{Benzaldehyde}}{\mathop{{{C}_{6}}{{H}_{5}}CHO}}\,\] > \[\underset{\text{Acetophenone}}{\mathop{{{C}_{6}}{{H}_{5}}COC{{H}_{3}}}}\,\] > \[\underset{\text{Benzophenone}}{\mathop{{{C}_{6}}{{H}_{5}}CO{{C}_{6}}{{H}_{5}}}}\,\]
Some important examples of nucleophilic addition reactions
Addition of HCN
\[R\overset{O}{\mathop{\overset{||}{\mathop{-C-}}\,}}\,H+HCN\xrightarrow{\overset{}{\mathop{O}}\,H}\] \[\underset{\text{Cyanohydrin}}{\mathop{R\underset{H}{\overset{\,\,\,\,\,OH}{\mathop{-\underset{|}{\overset{|}{\mathop{C}}}\,-}}}\,CN}}\,\]
\[\underset{\text{Benzaldehyde}}{\mathop{{{C}_{6}}{{H}_{5}}\overset{\,O}{\mathop{-\overset{||}{\mathop{C}}\,-}}\,H+HCN}}\,\xrightarrow{\overset{}{\mathop{O}}\,H}\] \[\underset{\text{Benzaldehyde cyanohydrin }}{\mathop{{{C}_{6}}{{H}_{5}}\underset{\,H}{\overset{\,\,\,\,\,OH}{\mathop{-\underset{|}{\overset{|}{\mathop{C}}}\,-}}}\,CN}}\,\]
Addition of sodium bisulphite
All types of aldehydes give addition reaction with this reagent.
\[R\overset{O}{\mathop{\overset{||}{\mathop{-C-}}\,}}\,H\ \xrightarrow{HS{{O}_{3}}Na}\underset{\begin{smallmatrix}\text{Adduct; white } \\\text{crystallin}\text{e in nature}\end{smallmatrix}}{\mathop{\underset{\,\,\,\,\,\,\,\,\,\,S{{O}_{3}}Na}{\overset{\,\,\,\,OH}{\mathop{\underset{|}{\overset{|}{\mathop{R-C-H}}}\,}}}\,}}\,\underset{HCHO}{\mathop{\xrightarrow{\overset{\oplus }{\mathop{H}}\,\ \text{or}\ \overset{\,\,}{\mathop{OH}}\,\ or}}}\,\ \overset{O}{\mathop{\overset{||}{\mathop{R-C-H}}\,}}\,\]
Only aliphatic methyl ketones give addition reaction with sodium bisulphite.
\[\overset{O\,\,\,\,\,\,\,}{\mathop{\overset{||\,\,\,\,\,\,\,\,\,\,}{\mathop{R-C-C{{H}_{3}}}}\,}}\,\xrightarrow{HS{{O}_{3}}Na}\underset{\text{product}}{\mathop{\underset{\text{Colourless crystalline }}{\mathop{\underset{\,\,\,\,S{{O}_{3}}Na}{\overset{OH\,}{\mathop{R\underset{|}{\overset{|}{\mathop{-C-}}}\,C{{H}_{3}}}}}\,}}\,}}\,\underset{HCHO}{\mathop{\xrightarrow{\overset{\oplus }{\mathop{H}}\,\ \text{or}\ \overset{\,\,}{\mathop{OH}}\,\ or}}}\,\ R-\overset{O}{\mathop{\overset{||}{\mathop{C}}\,}}\,-H\]
\[\overset{O}{\mathop{C{{H}_{3}}-C{{H}_{2}}\overset{||}{\mathop{-C-}}\,C{{H}_{2}}-C{{H}_{3}}}}\,\] and \[\overset{\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,O}{\mathop{C{{H}_{3}}-C{{H}_{2}}-C{{H}_{2}}\overset{||}{\mathop{-C-}}\,C{{H}_{3}}}}\,\]
\[\overset{\,O}{\mathop{\overset{\,\,\,||}{\mathop{{{C}_{6}}{{H}_{5}}-C-C{{H}_{3}}}}\,}}\,\] and \[\overset{\,\,\,\,\,\,\,\,\,\,\,\,\,\,O}{\mathop{C{{H}_{3}}-C{{H}_{2}}\overset{||}{\mathop{-C-}}\,C{{H}_{3}}}}\,\]
\[C{{H}_{3}}-C{{H}_{2}}-CHO\] and \[\overset{O}{\mathop{C{{H}_{3}}-C{{H}_{2}}\overset{||}{\mathop{-C-}}\,C{{H}_{2}}-C{{H}_{3}}}}\,\]
These two compounds can be separated from their mixture by the use of \[NaHS{{O}_{3}}\]. Higher aliphatic ketones and aromatic ketones do not react with \[NaHS{{O}_{3}}\].
Addition of alcohols : Carbonyl compounds give addition reaction with alcohols. This reaction is catalysed by acid and base. Nature of product depends on the catalyst.
Case I : Addition catalysed by base : In the presence of a base one equivalent of an alcohol reacts with only one equivalent of the carbonyl compound. The product obtained is called hemiacetal (in case of aldehyde) and hemiketal (in case of ketone). The reaction is reversible. There is always equilibrium between reactants and product.

Hemiacetals and hemiketals are \[\alpha -\]alkoxy alcohols.
Case II : Addition catalysed by acid : In the presence of an acid one equivalent of carbonyl compound reacts with two equivalents of alcohol. Product of the reaction is acetal (in case of aldehyde) or ketal (in case of ketone).
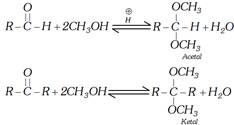
(i) Formation of acetals and ketals can be shown as follows:
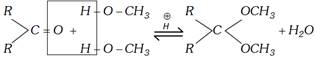
(ii) Acetals and ketals are gem dialkoxy compounds.
(iii) High yield of acetals or ketals are obtained if the water eliminated from the reaction is removed as it formed because the reaction is reversible.
(iv) Acetals and ketals can be transformed back to corresponding aldehyde or ketone in the presence of excess of water.
\[\underset{\text{Ketal}}{\mathop{\underset{\,\,\,\,\,\,\,\,\,OC{{H}_{3}}}{\overset{\,\,\,\,\,\,\,\,\,OC{{H}_{3}}}{\mathop{\underset{|}{\overset{|}{\mathop{R-C-R}}}\,}}}\,}}\,+\underset{\text{(Excess)}}{\mathop{{{H}_{2}}O}}\,\xrightarrow{\overset{\oplus }{\mathop{H}}\,}\ \overset{O}{\mathop{\overset{||}{\mathop{R-C-R}}\,}}\,\ +\ 2C{{H}_{3}}OH\]
This reaction is very useful reaction for the protection of carbonyl group which can be deprotected by hydrolysis. Glycol is used for this purpose. Suppose we want to carry out the given conversion by .
\[C{{H}_{3}}\overset{O}{\mathop{\overset{||}{\mathop{-C-}}\,}}\,C{{H}_{2}}-COO{{C}_{2}}{{H}_{5}}\xrightarrow{LiAl{{H}_{4}}}\] \[C{{H}_{3}}\overset{O}{\mathop{\overset{||}{\mathop{-C-}}\,}}\,C{{H}_{2}}-C{{H}_{2}}OH\]
This can be achieved by protection of ![]() group and then by deprotection
group and then by deprotection
Addition of Grignard reagents : Grignard reagents react with carbonyl compounds to give alcohols. Nature of alcohol depends on the nature of carbonyl compound.
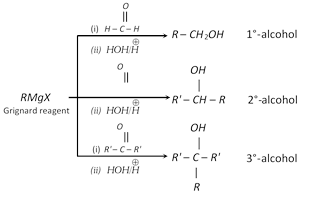
Addition of water : Carbonyl compounds react with water to give gem diols. This reaction is catalysed by acid. The reaction is reversible reaction.
\[\underset{\text{Ketone}}{\mathop{R\overset{O}{\mathop{\overset{||}{\mathop{-C-}}\,}}\,R'\ +\ HOH}}\,\underset{\text{Gemdiol}}{\mathop{R\underset{\,\,\,\,OH}{\overset{\,\,\,\,\,OH}{\mathop{\underset{|}{\overset{|}{\mathop{-C-}}}\,}}}\,R'}}\,\]
Gem diols are highly unstable compounds hence equilibrium favours the backward direction. The extent to which an aldehyde or ketone is hydrated depends on the stability of gem diol.
Stability of gem diols depend on the following factors:
(i) Steric hindrance by \[+I\] group around \[\alpha -\]carbon decreases the stability of gem diols. \[+I\] group decreases stability of gem diol and hence decreases extent of hydration.
(ii) Stability of gem diols mainly depends on the presence of \[-I\] group on \[\alpha -\]carbon. More is the \[-I\] power of the group more will be stability of gem diols.
(iii) Intramolecular hydrogen bonding increases stability of gem diols. \[-I\] groups present on carbon having gem diol group increases strength of hydrogen bond.
More is the strength of hydrogen bond more will be the stability of gem diol.
Addition of terminal alkynes : This reaction is known as ethinylation.
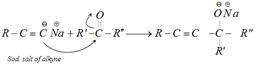
\[\xrightarrow{HOH/\overset{\oplus }{\mathop{H}}\,}R-C\equiv \underset{\text{alkynol}}{\mathop{C\underset{R'}{\overset{\,\,\,\,\,OH}{\mathop{-\underset{|}{\overset{|}{\mathop{C}}}\,-}}}\,R''}}\,\]
(2) Addition followed by elimination reactions : This reaction is given by ammonia derivatives \[(N{{H}_{2}}-Z)\].
(i) In nucleophilic addition reactions poor nucleophile such as ammonia and ammonia derivatives requires acid as catalyst.
(ii) If the attacking atom of the nucleophile has a lone pair of electrons in the addition product, water will be eliminated from the addition product. This is called a nucleophilic addition elimination.
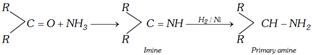
Primary amines and derivatives of ammonia react with carbonyl compounds to give adduct.
In adduct nucleophilic group has lone pair of electrons. It undergoes elimination to give product known as imine. An imine is a compound with a carbon-nitrogen double bond.
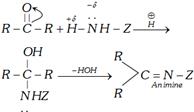
The overall reaction can be shown as follows
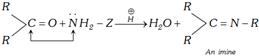
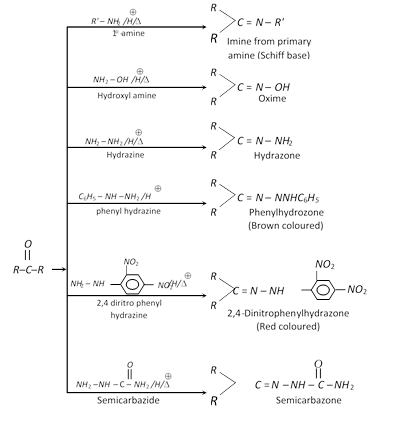
Different Imine formation with \[N{{H}_{2}}-Z\] is given below
Beckmann rearrangement : Ketoxime when treated with acid at \[{{0}^{o}}C\] it undergoes rearrangement known as Beckmann rearrangement.
Thus acid catalysed conversion of ketoximes to N-substituted amides is called Beckmann rearrangement. Acid catalyst used are proton acids\[({{H}_{2}}S{{O}_{4}},HCl,{{H}_{3}}P{{O}_{4}})\] and Lewis acids \[(PC{{l}_{5}},SOC{{l}_{2}},PhS{{O}_{2}}Cl,RCOCl,S{{O}_{3}},B{{F}_{3}}\] etc.)
\[\underset{\text{Acetophenoxime}}{\mathop{\underset{\,\,\,\,\,\,\,\,\,\,\,\,N-OH}{\mathop{{{C}_{6}}{{H}_{5}}\underset{||}{\mathop{-C-}}\,C{{H}_{3}}}}\,}}\,\underset{\text{(ii)}\ {{H}_{2}}O}{\mathop{\xrightarrow{\text{(i)}\ PC{{l}_{5}}}}}\,\overset{O\,\,\,\,\,\,\,\,\,\,\,\,\,\,}{\mathop{\underset{\text{N-phenylacetamide}}{\mathop{C{{H}_{3}}\overset{\,||}{\mathop{-C-}}\,NH-{{C}_{6}}{{H}_{5}}}}\,}}\,\]
\[\underset{\,\,\,\,\,\,\,\,\,\,\,N-OH\,\,\,}{\mathop{C{{H}_{3}}\underset{||}{\mathop{-C-}}\,{{C}_{6}}{{H}_{5}}}}\,\underset{\text{(ii)}\ {{H}_{2}}O}{\mathop{\xrightarrow{\text{(i)}\ PC{{l}_{5}}}}}\,\overset{O\,\,\,\,\,\,\,\,\,\,}{\mathop{\underset{\text{N-methyl acetamide}}{\mathop{{{C}_{6}}{{H}_{5}}\overset{||}{\mathop{-C-}}\,NH-C{{H}_{3}}}}\,}}\,\]
In short product of the rearrangement can be obtained as follows:
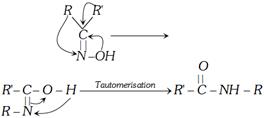
(3) Oxidation of carbonyl compounds
(i) Oxidation by mild oxidising agents : Mild oxidising agents oxidise only aldehydes into carboxylic acids. They do not oxidises ketones. Main oxidising agents are:
(a) Fehling solution : It is a mixture of two Fehling solution: Fehling solution No. 1 : It contains \[CuS{{O}_{4}}\] solution and NaOH.
Fehling solution No. 2 : It contains sodium potassium tartrate. (Roschelle salt).
(b) Benedict's solution : This solution contains \[CuS{{O}_{4}},NaOH\]and sodium or potassium citrate.
Benedict's solution and Fehling solutions are used as a reagent for the test of sugar (glucose) in blood sample.
(c) Tollens reagent : Tollens reagent is ammonical silver nitrate solution. Its reacting species is \[A{{g}^{\oplus }}\].
\[R-CHO+A{{g}^{\oplus }}\underset{reaction}{\mathop{\xrightarrow{\text{Redox}}}}\,RCOOH+Ag\] (as silver mirror)
\[C{{H}_{2}}=CH-CHO+A{{g}^{\oplus }}\xrightarrow{{}}C{{H}_{2}}=CH-COOH+Ag\]
In this reaction the oxidation no. of Ag varies from +1 to 0.
\[{{C}_{5}}{{H}_{11}}{{O}_{5}}CHO+C{{u}_{2}}O\] (or) \[A{{g}_{2}}O\xrightarrow{{}}\ \underset{\text{Gluconic acid}}{\mathop{{{C}_{5}}{{H}_{11}}{{O}_{5}}COOH}}\,\]
Fructose contain ![]() (keto) group yet give positive test with Fehling solution due to presence of a-hydroxyl keto group. Tollens reagent also gives positive test with terminal alkynes and HCOOH.
(keto) group yet give positive test with Fehling solution due to presence of a-hydroxyl keto group. Tollens reagent also gives positive test with terminal alkynes and HCOOH.
(d) Reaction with mercuric chloride solution :
\[\underset{O}{\mathop{\underset{||}{\mathop{R-C-H}}\,}}\,+HgC{{l}_{2}}+{{H}_{2}}O\xrightarrow{{}}\underset{O\,\,\,\,}{\mathop{\underset{||\,\,\,\,\,\,}{\mathop{R-C-OH}}\,}}\,+HCl+\underset{\text{(White)}}{\mathop{H{{g}_{2}}C{{l}_{2}}(\downarrow )}}\,\]
\[\underset{O}{\mathop{\underset{||}{\mathop{R-C-H}}\,}}\,+H{{g}_{2}}C{{l}_{2}}+{{H}_{2}}O\xrightarrow{{}}\underset{O\,\,\,}{\mathop{\underset{||\,\,\,\,}{\mathop{\ R-C-OH}}\,}}\,+HCl+\underset{\text{(Black)}}{\mathop{Hg(\downarrow )}}\,\]
(e) Schiff's reagent : Megenta solution\[\xrightarrow{S{{O}_{2}}}\] colourless solution \[\xrightarrow{C{{H}_{3}}CHO}\] pink colour restored (In cold).
(ii) Oxidation by strong oxidising agents : Main strong oxidising agents are \[KMn{{O}_{4}}/O{{H}^{}}/\Delta ,\] \[KMn{{O}_{4}}/{{H}^{\oplus }}/\Delta ,\] \[{{K}_{2}}C{{r}_{2}}{{O}_{7}}/{{H}^{\oplus }}/\Delta \ \] and conc \[HN{{O}_{3}}/\Delta \]. These agents oxidise aldehydes as well as ketones.
(a) Oxidation of aldehydes : Aldehydes are oxidised into corresponding acids.
\[\underset{C=n}{\mathop{RCHO}}\,\xrightarrow{[O]}\underset{C=n}{\mathop{RCOOH}}\,\]
\[{{C}_{6}}{{H}_{5}}CHO\xrightarrow{KMn{{O}_{4}}/\overset{}{\mathop{O}}\,H/\Delta }{{C}_{6}}{{H}_{5}}COOH\]
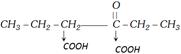
(b) Oxidation of ketones : Ketones undergo oxidation only in drastic conditions. During the oxidation of ketones there is breaking of carbon-carbon bond between \[\alpha -\]carbon and carbonyl carbon. In this process both carbons convert into carboxylic groups. This leads to the formation of two moles of monocarboxylic acids.
Case I : Oxidation of symmetrical ketones
![]()
\[C{{H}_{2}}-C{{H}_{2}}-C{{H}_{3}}\xrightarrow{[O]}\]
\[\underset{\text{Total number of }C=\text{4}+\text{3}=\text{7}}{\mathop{\underset{\text{C}=\text{4}}{\mathop{C{{H}_{3}}-C{{H}_{2}}-C{{H}_{2}}-COOH}}\,+\underset{\text{C}=\text{3}\,\,\,\,\,\,\,\,\,\,\,\,}{\mathop{C{{H}_{3}}-C{{H}_{2}}-COOH}}\,}}\,\]
Thus number of carbons in any product is less than the number of carbons in ketone.
Case II : Oxidation of unsymmetrical ketones : In case of unsymmetrical ketones \[\alpha -\]carbon whose bond breaks always belongs to the alkyl group which has more number of carbons. This rule is known as Popoff’s rule.
\[\xrightarrow{[O]}C{{H}_{3}}-C{{H}_{2}}-COOH+C{{H}_{3}}-C{{H}_{2}}-COOH\]
Case III : Oxidation of cyclic ketones : Formation of dibasic acid takes place from cyclic ketones. In this case the number of carbons in ketone and dibasic carboxylic acid is always same.
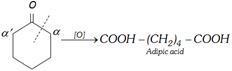
If both \[\alpha -\]carbons are not identical then bond breaking takes place between carbonyl carbon and the \[\alpha -\]carbon which has maximum number of hydrogens.
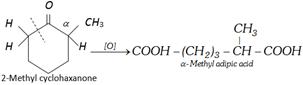
(iii) Miscellaneous oxidation
(a) Haloform Reaction
\[\underset{\alpha -\text{methyl carbonyl}}{\mathop{R\overset{O}{\mathop{\overset{||}{\mathop{-C-}}\,}}\,C{{H}_{3}}}}\,\underset{\text{(ii)}\ \overset{\oplus }{\mathop{H}}\,\ }{\mathop{\xrightarrow{\text{(i)}\ {{\text{X}}_{\text{2}}}/\overset{}{\mathop{OH}}\,}}}\,\ RCOOH+CH{{X}_{3}}\]
(b) Oxidation at \[\alpha -C{{H}_{2}}\] or \[C{{H}_{3}}\] by \[Se{{O}_{2}}:Se{{O}_{2}}\] oxidises \[\alpha -C{{H}_{2}}-\]group into keto group and \[\alpha -C{{H}_{3}}-\]group into aldehydic group.
In this oxidation reactivity of \[C{{H}_{2}}\] is more than the \[C{{H}_{3}}\]group and Oxidation is regio selective in nature.
\[C{{H}_{3}}-CHO\xrightarrow{Se{{O}_{2}}}\underset{\text{Glyoxal}}{\mathop{CHO-CHO}}\,\]:
\[\overset{O}{\mathop{\overset{||}{\mathop{C{{H}_{3}}-C-C{{H}_{3}}}}\,}}\,\xrightarrow{Se{{O}_{2}}}\ \underset{\text{Methylglyoxal}}{\mathop{C{{H}_{3}}\overset{O}{\mathop{\overset{||}{\mathop{-C-}}\,}}\,CHO}}\,\]
(c) Oxidation by organic peracids : Organic peracids oxidise aldehydes into carboxylic acids and ketones into esters. This oxidation is known as Baeyer – Villiger oxidation.
\[\overset{O}{\mathop{\overset{||}{\mathop{R-C-{R}'}}\,}}\,\xrightarrow{{{C}_{6}}{{H}_{5}}COOOH}\overset{O\,\,\,\,\,\,\,\,}{\mathop{\overset{||\,\,\,\,\,\,\,\,\,\,\,\,\,}{\mathop{R-C-O-{R}'}}\,}}\,\]
In case of aldehyde there is insertion of atomic oxygen (obtained from peracid) between carbonyl carbon and hydrogen of carbonyl carbon.
In case of ketone, insertion of oxygen takes place between carbonyl carbon and a-carbon. Thus the product is ester. This is one of the most important reaction for the conversion of ketones into esters.
\[R-\overset{O}{\mathop{\overset{||}{\mathop{C}}\,}}\,-\overset{O}{\mathop{\overset{||}{\mathop{C}}\,}}\,-R\,\,\xrightarrow{{{C}_{6}}{{H}_{5}}COOOH}\,\,R-\underset{O}{\mathop{\underset{||}{\mathop{C}}\,}}\,-O-\underset{O}{\mathop{\underset{||}{\mathop{C}}\,}}\,-R\]
Example :
\[C{{H}_{3}}-CO-C{{H}_{2}}-C{{H}_{3}}\xrightarrow{[O]}C{{H}_{3}}-COOH+HOOCC{{H}_{3}}\]
(d) Baeyer- villiger oxidation :
\[\underset{O}{\mathop{\underset{||}{\mathop{H-C-H}}\,}}\,\ +\ \overset{H}{\mathop{\overset{|}{\mathop{O}}\,}}\,\,-O\underset{O}{\mathop{\underset{||}{\mathop{-C-}}\,}}\,H\xrightarrow{{}}\underset{O\,\,\,\,}{\mathop{H\underset{\,||\,}{\mathop{-C-}}\,OH}}\,\]
\[C{{H}_{3}}\underset{O}{\mathop{-\underset{||}{\mathop{C}}\,-}}\,H+\overset{H}{\mathop{\overset{|}{\mathop{O}}\,}}\,-O\underset{O}{\mathop{\underset{||}{\mathop{-C-}}\,}}\,H\xrightarrow{{}}C{{H}_{3}}\underset{O}{\mathop{\underset{\,||\,}{\mathop{-C-}}\,}}\,OH\]
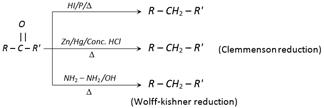
(4) Reduction of carbonyl compounds
(i) Reduction of group into\[-C{{H}_{2}}\] – group : Following three reagents reduce carbonyl group into \[-C{{H}_{2}}-\] groups: (a) \[HI/P/\Delta \] (b) \[Zn/Hg/Conc.\ HCl\] and (c) \[N{{H}_{2}}-N{{H}_{2}}/\overset{\,\,\,\,\,\,}{\mathop{OH}}\,\].
(ii) Reduction of carbonyl compounds into hydroxy compounds : Carbonyl group converts into \[-CHOH-\]group by \[LiAl{{H}_{4}},NaB{{H}_{4}},Na/{{C}_{2}}{{H}_{5}}OH\] and aluminium isopropoxide.
\[R-CHO\ \underset{\text{(iii) Aluminium isopropoxide}}{\mathop{\underset{\text{(ii) }NaB{{H}_{\text{4}}}}{\mathop{\xrightarrow{\ \ \ \ \ \text{(i) LiAl}{{\text{H}}_{\text{4}}}\ \ \ \ \ }}}\,}}\,R-C{{H}_{2}}OH\]
\[\overset{O}{\mathop{\overset{||}{\mathop{R-C-R}}\,}}\,'\underset{\text{(iii) Aluminium isopropoxide}}{\mathop{\underset{\text{(ii)}\ NaB{{H}_{4}}}{\mathop{\xrightarrow{\ \ \ \ \ \text{(i) }LiAl{{H}_{4}}\ \ \ \ \ }}}\,}}\,\overset{OH}{\mathop{\overset{|\,\,\,\,\,}{\mathop{R-CH-R}}\,'}}\,\]
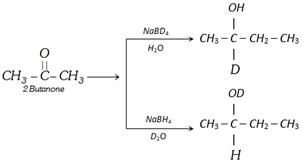
\[NaB{{H}_{4}}\] is regioselective reducing agent because it reduced only. CHO in the presence of other reducible group.
Example :
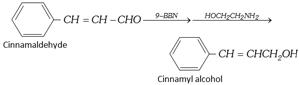
\[\underset{\text{Crotonaldehyde}}{\mathop{C{{H}_{3}}-CH=CH-CHO}}\,\xrightarrow{NaB{{H}_{4}}}\underset{\text{Crotonyl alcohol}}{\mathop{C{{H}_{3}}-CH=CH-C{{H}_{2}}OH}}\,\]
Hydride ion of \[NaB{{H}_{4}}\] attack on carbonyl carbon during reduction.
Example :
\[\underset{2\text{-Butanone}}{\mathop{C{{H}_{3}}-\overset{O}{\mathop{\overset{||}{\mathop{C}}\,}}\,-C{{H}_{3}}}}\,\underset{{{D}_{2}}O}{\mathop{\xrightarrow{NaB{{D}_{4}}}}}\,C{{H}_{3}}\overset{\,\,\,OD}{\mathop{\overset{|}{\mathop{-\underset{D}{\mathop{\underset{|}{\mathop{C}}\,}}\,-}}\,}}\,C{{H}_{2}}-C{{H}_{3}}\]
(iii) Reductive amination : In this reduction \[-CO-\]group converts into \[-CH-N{{H}_{2}}\]group
(iv) Reduction of ketones by Mg or Mg/Hg : In this case ketones undergo reduction via coupling reaction and product is vic cis diol.
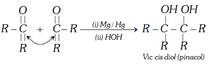
When this reaction is carried out in the presence of \[Mg/Hg/TiC{{l}_{4}}\], the product is vic trans diol.

(v) Reduction of benzaldehyde by \[Na/{{C}_{2}}{{H}_{5}}OH\]: Benzaldehyde undergoes reduction via coupling reaction and product is vic diol.
\[{{C}_{6}}{{H}_{5}}-\underset{H}{\overset{O}{\mathop{\underset{|}{\overset{||}{\mathop{C}}}\,}}}\,+\underset{H}{\overset{O}{\mathop{\underset{|}{\overset{||}{\mathop{C}}}\,}}}\,-{{C}_{6}}{{H}_{5}}\underset{\text{(ii) }HOH}{\mathop{\xrightarrow{\text{(i) }Na/{{C}_{2}}{{H}_{5}}OH}}}\,\]
\[\underset{vic\ diol}{\mathop{\overset{\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,OH}{\mathop{{{C}_{6}}{{H}_{5}}\overset{|\,}{\mathop{-\ CH\ }}\,-}}\,\overset{OH\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,}{\mathop{\overset{|}{\mathop{C}}\,H-{{C}_{6}}{{H}_{5}}}}\,}}\,\] (Bouveault-blanc reaction)
Example :
(vi) Hydrazones when treated with base like alkoxide give hydrocarbon (Wolf – Kishner reduction).
\[\overset{O}{\mathop{\overset{||}{\mathop{R-C-R}}\,}}\,'\xrightarrow{N{{H}_{2}}N{{H}_{2}}}\underset{\text{Hydrazone}}{\mathop{\overset{\,\,\,\,\,\,\,\,\,\,\,N\mathbf{.}N{{H}_{2}}}{\mathop{\overset{||}{\mathop{R-C-R}}\,'}}\,}}\,\underset{\Delta }{\mathop{\xrightarrow{RONa}}}\,R-C{{H}_{2}}-R\]
(vii) Schiff's base on reduction gives secondary amines.
\[\underset{\text{Aldehyde}}{\mathop{R-CH=O}}\,\xrightarrow{R'N{{H}_{2}}}\underset{\text{Schiff }\!\!'\!\!\text{ s base}}{\mathop{R-CH=NR'}}\,\xrightarrow{{{H}_{2}}/Ni}\underset{\text{Secondary amine}}{\mathop{R-C{{H}_{2}}NHR}}\,\]
(5) Reactions due to \[\alpha -\]hydrogen
(i) Acidity of \[\alpha -\]hydrogens :
(a) \[\alpha -\]hydrogen of carbonyl compounds are acidic in character due to the presence of the electron withdrawing \[-CO-\] group.
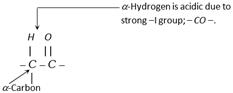
(b) Thus carbonyl compounds having \[\alpha -\]hydrogen convert into carbanions in the presence of base. This carbanion is stabilised by delocalisation of negative charge.
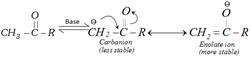
(c) The acidity of \[\alpha -\]hydrogen is more than ethyne. pKa value of aldehydes and ketones are generally 19 – 20 where as pKa value of ethyne is 25.
(d) Compounds having active methylene or methyne group are even more acidic than simple aldehydes and ketones.
\[\underset{\alpha \text{-phenyl acetone}}{\mathop{{{C}_{6}}{{H}_{5}}-\overset{\alpha \,\,\,}{\mathop{\underline{C{{H}_{2}}}}}\,\overset{O}{\mathop{\overset{||}{\mathop{-C-}}\,}}\,C{{H}_{3}}}}\,\] \[pKa=15.9\]
\[\underset{\alpha \text{-benzoyl acetone}}{\mathop{{{C}_{6}}{{H}_{5}}\overset{O}{\mathop{\overset{||}{\mathop{-C-}}\,}}\,\overset{\alpha }{\mathop{\underline{C{{H}_{2}}}}}\,\overset{O}{\mathop{\overset{||}{\mathop{-C-}}\,}}\,C{{H}_{3}}}}\,\] \[pKa=8.5\]
(ii) Halogenation : Carbonyl compounds having a-hydrogens undergo halogenation reactions. This reaction is catalysed by acid as well as base.
(a) Acid catalysed halogenation : This gives only monohalo derivative.
\[\underset{\text{Acetone}}{\mathop{\overset{O}{\mathop{C{{H}_{3}}\overset{||}{\mathop{-C-}}\,C{{H}_{3}}}}\,}}\,\xrightarrow{B{{r}_{2}}/C{{H}_{3}}COOH}\underset{\alpha -\text{bromo acetone}}{\mathop{C{{H}_{3}}-\overset{O}{\mathop{\overset{||}{\mathop{C}}\,}}\,-C{{H}_{2}}Br}}\,\]
(b) Base catalysed halogenation : In the presence of base all \[\alpha -\]hydrogens of the same carbon is replaced by halogens.
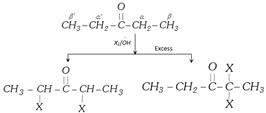
Carbonyl compounds having three a-hydrogens give haloform reaction.
\[R-\overset{O}{\mathop{\overset{||}{\mathop{C}}\,}}\,-C{{H}_{3}}\xrightarrow{{{X}_{2}}/\overset{\,\,}{\mathop{OH}}\,}R-\overset{O}{\mathop{\overset{||}{\mathop{C}}\,}}\,-C{{X}_{3}}\xrightarrow{\overset{\,\,\,\,\,\,\,}{\mathop{OH}}\,}\ \overset{\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,}{\mathop{RCOO}}\,\ +CH{{X}_{3}}\]
(iii) Deuterium exchange reaction : Deuterium exchange reaction is catalysed by acid \[({{D}^{\oplus }})\] as well as base \[(\overset{\,\,\,\,\,\,}{\mathop{OD}}\,)\]. In both the cases all the hydrogens on only one a-carbon is replaced by D.
\[R-\overset{O}{\mathop{\overset{||}{\mathop{C}}\,}}\,-C{{H}_{2}}-R\xrightarrow{{{D}_{2}}O/\overset{\,\,}{\mathop{OD}}\,}R-\overset{O}{\mathop{\overset{||}{\mathop{C}}\,}}\,-C{{D}_{2}}-R\]
\[R-\overset{O}{\mathop{\overset{||}{\mathop{C}}\,}}\,-C{{H}_{2}}-R\xrightarrow{{{D}_{2}}O/\overset{\,\oplus }{\mathop{D}}\,}R-\overset{O}{\mathop{\overset{||}{\mathop{C}}\,}}\,-C{{D}_{2}}-R\]
(iv) Racemisation : Ketones whose carbon is chiral undergo Racemisation in the presence of acid as well as base.
\[\underset{2-\text{methyl-1-phenyl-1-one}}{\mathop{{{C}_{6}}{{H}_{5}}\overset{\,\,\,O}{\mathop{-\overset{||}{\mathop{C}}\,}}\,\underset{H\,\,\,\,\,\,\,\,\,\,\,}{\overset{C{{H}_{3\,\,\,\,\,\,\,\,\,\,\,}}}{\mathop{-\underset{|}{\overset{|}{\mathop{C}}}\,-{{C}_{2}}{{H}_{5}}}}}\,}}\,\underset{\overset{\,\,\,\,\,\,}{\mathop{OH}}\,}{\mathop{\xrightarrow{{{H}^{\oplus }}\text{ or}}}}\,\underset{\text{Racemic mixture}}{\mathop{{{C}_{6}}{{H}_{5}}-\overset{O}{\mathop{\overset{||}{\mathop{C}}\,}}\,\underset{H\,\,\,\,\,\,\,\,\,\,\,}{\overset{C{{H}_{3\,\,\,\,\,\,\,\,\,\,}}}{\mathop{-\underset{|}{\overset{|}{\mathop{C}}}\,-{{C}_{2}}{{H}_{5}}}}}\,\,\,\,\,}}\,\,\]
\[+\underset{{}}{\mathop{\underset{\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,H}{\overset{\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,C{{H}_{3}}}{\mathop{{{C}_{2}}{{H}_{5}}-\underset{|}{\overset{|}{\mathop{C}}}\,}}}\,-\overset{O}{\mathop{\overset{||}{\mathop{C}}\,}}\,-{{C}_{6}}{{H}_{5}}}}\,\]
(v) Alkylation : Carbonyl compounds having \[\alpha -\]hydrogens undergo alkylation reaction with RX in the presence of base. This reaction is \[{{S}_{{{N}^{2}}}}\] reaction. The best result is obtained with \[C{{H}_{3}}-X\]. Other halides undergo elimination in the presence of strong base.

(vi) Wittig reaction : Aldehyde and ketones undergo the wittig reaction to form alkenes.
\[P{{h}_{3}}P=C{{H}_{2}}+\underset{\text{or ketone}}{\mathop{\underset{\text{Aldehyde}}{\mathop{>C=O}}\,}}\,\xrightarrow{{}}\underset{\text{alkene}}{\mathop{>C}}\,=C{{H}_{2}}+\underset{\text{Phosphonium oxide}}{\mathop{\underset{\text{Triphenyl}}{\mathop{P{{h}_{3}}P=O}}\,}}\,\]
\[P{{h}_{3}}P=CH{{R}^{1}}\ +\ \underset{O\,\,\,\,\,\,\,\,}{\mathop{\underset{||\,\,\,\,\,\,\,\,\,\,\,\,\,}{\mathop{CH{{R}^{2}}}}\,}}\,\xrightarrow{{}}\underset{\,\,\,\,\,\,\,{{O}^{}}-CH{{R}^{2}}}{\mathop{\underset{\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,|}{\mathop{P{{h}_{3}}{{P}^{\oplus }}-CH{{R}^{1}}}}\,}}\,\xrightarrow{{}}\]
\[\underset{\,\,\,\,\,\,\,O\,-\,CH{{R}^{2}}}{\mathop{P{{h}_{3}}\underset{|\,\,\,}{\mathop{P}}\,-\underset{|\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,}{\mathop{CH{{R}^{1}}}}\,}}\,\xrightarrow{{}}P{{h}_{3}}\underset{O}{\mathop{\underset{||}{\mathop{P}}\,}}\,+\underset{CH{{R}^{2}}}{\mathop{\underset{||\ \ \ \ \ \ \ \,\,}{\mathop{CH{{R}^{1}}}}\,}}\,\]
(6) Condensation reaction of carbonyl compounds : Nucleophilic addition reaction of compounds having carbonyl group with those compounds which have at least one acidic hydrogen at \[\alpha -\]carbon is known as condensation reaction. In this addition reaction :
Substrate is always an organic compound having a carbonyl group, e.g.
\[\overset{O}{\mathop{\overset{||}{\mathop{H-C-H}}\,}}\,,\] \[\overset{\,\,\,\,\,\,\,O}{\mathop{\overset{\,\,\,\,\,\,\,\,\,||}{\mathop{{{C}_{6}}{{H}_{5}}-C-H}}\,}}\,,\] \[\overset{O}{\mathop{\overset{||}{\mathop{R-C-H}}\,}}\,,\] \[\overset{O}{\mathop{\overset{||}{\mathop{R-C-R}}\,}}\,\] etc.
Addition always takes place on the carbonyl group.
Reagents of the condensation reaction are also organic compounds having at least one hydrogen on \[\alpha -\]carbon and \[\alpha -\]carbon should have –I group, e.g.
\[\overset{\alpha }{\mathop{C{{H}_{3}}}}\,-N{{O}_{2}},\] \[C{{H}_{3}}\underset{C{{H}_{3}}}{\mathop{\underset{|\,\,\,\,\,\,\,\,}{\overset{\alpha \,\,\,\,\,}{\mathop{-CH-}}}\,}}\,CHO,\] \[C{{H}_{3}}\overset{\alpha \ \ \ \ \ }{\mathop{-C{{H}_{2}}-}}\,CN\]
If substrate and reagent both are carbonyl compounds then one should have at least one \[\alpha -\]hydrogen and other may or may not have \[\alpha -\]hydrogen.
Condensation reaction always takes place in the presence of acid or base as catalyst. Best result is obtained with base at lower temp.
\[\overset{O}{\mathop{\overset{||}{\mathop{R-C-R}}\,}}\,\ +\ C{{H}_{3}}-Z\underset{\overset{\,\,}{\mathop{OH}}\,}{\mathop{\xrightarrow{{{H}^{\oplus }}\text{ or}}}}\,\ \underset{R\,\,\,\,\,}{\overset{OH\,}{\mathop{R\underset{|}{\overset{|}{\mathop{-{}_{\alpha }C\ \ \,-}}}\,\ \ \underset{\beta }{\mathop{C}}\,{{H}_{2}}}}}\,-Z\]
Condensation is carried out at lower temperature \[(\le 20{}^\circ C)\] because product of the reaction is alcohol which has strong \[-I\] group at \[\beta -\]carbon.
Such type of alcohols are highly reactive for dehydration. They undergo dehydration in the presence of acid as well as base even at \[{{25}^{o}}C\]. They also undergo elimination even on strong heating.
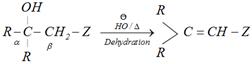
(i) Aldol condensation
(a) This reaction takes place between two molecules of carbonyl compounds; one molecule should have at least two \[\alpha -\]hydrogen atoms. In this reaction best result is obtained when
Both molecule are the same or
One should have no \[\alpha -\]hydrogen atom and other should have at least two \[\alpha -\]hydrogens.
(b) These reactions are practical when base is NaOH and reaction temperature is high \[(\ge 100{}^\circ )\].
(c) The reaction is two step reaction. First step is aldol formation and second step is dehydration of aldol.
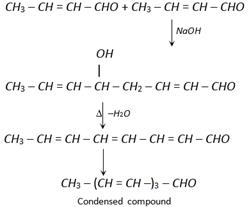
\[C{{H}_{3}}-CHO+C{{H}_{3}}-CHO\underset{\Delta }{\mathop{\xrightarrow{NaOH/\overset{\,\,}{\mathop{OH}}\,}}}\,\left[ \overset{OH\,\,\,\,\,\,\,\,\,\,\,\,}{\mathop{C{{H}_{3}}-\overset{|\,\,\,\,\,\,}{\mathop{CH}}\,-C{{H}_{2}}-CHO}}\, \right]\]
\[\xrightarrow{\text{Dehydration}}\underset{a,\ \beta -\text{unsaturated aldehyde}}{\mathop{C{{H}_{3}}-CH=CH-CHO}}\,\]
Due to hyper-conjugation in crotonaldehyde further condensation give conjugated alkene carbonyl compound.
Mechanism : \[{{C}_{6}}{{H}_{5}}-CHO+C{{H}_{3}}-CHO\xrightarrow{\overset{\,\,}{\mathop{OH}}\,/\Delta }\]
![]()
Step I : \[\overset{\,\,\,\,\,\,\,}{\mathop{HO}}\,\,+H-C{{H}_{2}}-CHO\]
\[HOH+\left[ \overset{\,\,\,\,\,\,\,\,\,}{\mathop{C{{H}_{2}}}}\,\overset{O}{\mathop{-\overset{||}{\mathop{C}}\,-}}\,H\overset{{}}{\longleftrightarrow}C{{H}_{2}}\overset{}{\mathop{\overset{O}{\mathop{=\overset{|}{\mathop{C}}\,-}}\,}}\,H \right]\]
Step II : \[{{C}_{6}}{{H}_{5}}\underset{\,\,\,H}{\overset{\,\,\,O}{\mathop{-\underset{|}{\overset{||}{\mathop{C}}}\,}}}\,\ +\ \overset{\,\,\,\,\,\,\,\,}{\mathop{C{{H}_{2}}}}\,-CHO\xrightarrow{{}}\]
\[{{C}_{6}}{{H}_{5}}\underset{\,H}{\overset{\,\overset{}{\mathop{O}}\,}{\mathop{-\underset{|}{\overset{|}{\mathop{C}}}\,-}}}\,C{{H}_{2}}-CHO\]
\[\xrightarrow{HOH}\underset{H\,\,\,\,\,\,\,\,\,\,\,\,}{\overset{OH\,\,\,\,\,\,\,\,}{\mathop{{{C}_{6}}{{H}_{5}}-\underset{|}{\overset{|}{\mathop{C}}}\,-C{{H}_{2}}-CHO}}}\,\ +\ \overset{\,\,\,\,\,\,}{\mathop{OH}}\,\]
Step III :
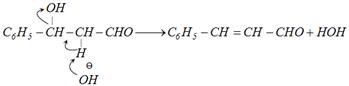
In aldol condensation, dehydration occurs readily because the double bond that forms is conjugated, both with the carbonyl group and with the benzene ring. The conjugation system is thereby extended.
Crossed aldol condensation : Aldol condensation between two different aldehydes or two different ketones or one aldehyde and another ketone provided at least one of the components have a-hydrogen atom gives different possible product
(a) \[\underset{\text{Ethanal}}{\mathop{C{{H}_{3}}CHO}}\,+\underset{\text{Propanal}}{\mathop{C{{H}_{3}}-C{{H}_{2}}-CHO}}\,\xrightarrow{\text{dil }NaOH}\]
\[C{{H}_{3}}-\overset{OH}{\mathop{\overset{|\,\ \ \ }{\mathop{CH}}\,}}\,-\overset{C{{H}_{3}}}{\mathop{\overset{|\,\ \ \ \,\,\,\,}{\mathop{CH\,\,}}\,}}\,-CHO+C{{H}_{3}}-C{{H}_{2}}-CHOH-C{{H}_{2}}-CHO\]
However crossed aldol condensation is important when only it the components has \[\alpha -\]hydrogen atom.
\[C{{H}_{2}}O+C{{H}_{3}}CHO\xrightarrow{{}}\underset{\text{(3-hydroxy propanal)}}{\mathop{\underset{OH\,}{\mathop{\underset{|\,\,\,\,\,\,\,\,\,\,}{\mathop{C{{H}_{2}}}}\,}}\,-C{{H}_{2}}-CHO}}\,\underset{-{{H}_{2}}O}{\mathop{\xrightarrow{\Delta }}}\,\underset{\text{(Acrolein)}}{\mathop{C{{H}_{2}}=CH-CHO}}\,\]
Intra molecular aldol condensation : One molecule Intramolecular condensation give aldol compounds
Example :
![]()
(ii) Claisen – Schmidt reaction : Crossed aldol condensation between aromatic aldehyde and aliphatic ketone or mixed ketone is known as Claisen – Schmidt reaction. Claisen – Schmidt reactions are useful when bases such as sodium hydroxide are used because under these conditions ketones do not undergo self condensation. Some examples of this reaction are :
\[{{C}_{6}}{{H}_{5}}CHO+C{{H}_{3}}\overset{O}{\mathop{\overset{||}{\mathop{-C-}}\,}}\,C{{H}_{3}}\overset{\overset{\,\,}{\mathop{OH}}\,}{\mathop{\xrightarrow[100{}^\circ C]{}}}\,\underset{\text{4}-\text{Phenyl}-3-\text{buten-2-one }}{\mathop{{{C}_{6}}{{H}_{5}}-CH=CH\overset{O}{\mathop{\overset{||\,}{\mathop{-C-}}\,}}\,C{{H}_{3}}}}\,\]
CH=CH-CHO}}\,\] Due to hyper-conjugation in crotonaldehyde further condensation give conjugated alkene carbonyl compound. \[\] Mechanism : \[{{C}_{6}}{{H}_{5}}-CHO+C{{H}_{3}}-CHO\xrightarrow{\overset{{\mathrm O}-\,\,\,}{\mathop{OH}}\,/\Delta }\] \[{{C}_{6}}{{H}_{5}}-CH=CH-CHO+HOH\] Step I : \[HOH+\left[ \overset{{\mathrm O}-\,\,\,\,\,\,\,\,\,}{\mathop{C{{H}_{2}}}}\,\overset{O}{\mathop{-\overset{||}{\mathop{C}}\,-}}\,H\overset{{}}{\longleftrightarrow}C{{H}_{2}}\overset{{\mathrm O}-\,}{\mathop{\overset{O}{\mathop{=\overset{|}{\mathop{C}}\,-}}\,}}\,H \right]\] Step II : \[{{C}_{6}}{{H}_{5}}\underset{\,\,\,H}{\overset{\,\,\,O}{\mathop{-\underset{|}{\overset{||}{\mathop{C}}}\,}}}\,\ +\ \overset{{\mathrm O}-\,\,\,\,\,\,\,}{\mathop{C{{H}_{2}}}}\,-CHO\xrightarrow{{}}\] \[{{C}_{6}}{{H}_{5}}\underset{\,H}{\overset{\,\overset{{\mathrm O}-\,}{\mathop{O}}\,}{\mathop{-\underset{|}{\overset{|}{\mathop{C}}}\,-}}}\,C{{H}_{2}}-CHO\]\[\xrightarrow{HOH}\underset{H\,\,\,\,\,\,\,\,\,\,\,\,}{\overset{OH\,\,\,\,\,\,\,\,}{\mathop{{{C}_{6}}{{H}_{5}}-\underset{|}{\overset{|}{\mathop{C}}}\,-C{{H}_{2}}-CHO}}}\,\ +\ \overset{{\mathrm O}-\,\,\,\,\,\,}{\mathop{OH}}\,\] Step III : In aldol condensation, dehydration occurs readily because the double bond that forms is conjugated, both with the carbonyl group and with the benzene ring. The conjugation system is thereby extended. Crossed aldol condensation : Aldol condensation between two different aldehydes or two different ketones or one aldehyde and another ketone provided at least one of the components have a-hydrogen atom gives different possible product (a) \[\underset{\text{Ethanal}}{\mathop{C{{H}_{3}}CHO}}\,+\underset{\text{Propanal}}{\mathop{C{{H}_{3}}-C{{H}_{2}}-CHO}}\,\xrightarrow{\text{dil }NaOH}\] \[C{{H}_{3}}-\overset{OH}{\mathop{\overset{|\,\ \ \ }{\mathop{CH}}\,}}\,-\overset{C{{H}_{3}}}{\mathop{\overset{|\,\ \ \ \,\,\,\,}{\mathop{CH\,\,}}\,}}\,-CHO+C{{H}_{3}}-C{{H}_{2}}-CHOH-C{{H}_{2}}-CHO\] However crossed aldol condensation is important when only it the components has a-hydrogen atom. \[C{{H}_{2}}O+C{{H}_{3}}CHO\xrightarrow{{}}\underset{\text{(3-hydroxy propanal)}}{\mathop{\underset{OH\,}{\mathop{\underset{|\,\,\,\,\,\,\,\,\,\,}{\mathop{C{{H}_{2}}}}\,}}\,-C{{H}_{2}}-CHO}}\,\underset{-{{H}_{2}}O}{\mathop{\xrightarrow{\Delta }}}\,\underset{\text{(Acrolein)}}{\mathop{C{{H}_{2}}=CH-CHO}}\,\] Intra molecular aldol condensation : One molecule Intramolecular condensation give aldol compounds Example : (ii) Claisen ? Schmidt reaction : Crossed aldol condensation between aromatic aldehyde and aliphatic ketone or mixed ketone is known as Claisen ? Schmidt reaction. Claisen ? Schmidt reactions are useful when bases such as sodium hydroxide are used because under these conditions ketones do not undergo self condensation. Some examples of this reaction are : \[{{C}_{6}}{{H}_{5}}CHO+C{{H}_{3}}\overset{O}{\mathop{\overset{||}{\mathop{-C-}}\,}}\,C{{H}_{3}}\overset{\overset{{\mathrm O}-\,\,}{\mathop{OH}}\,}{\mathop{\xrightarrow[100{}^\circ C]{}}}\,\underset{\text{4}-\text{Phenyl}-3-\text{buten-2-one }}{\mathop{{{C}_{6}}{{H}_{5}}-CH=CH\overset{O}{\mathop{\overset{||\,}{\mathop{-C-}}\,}}\,C{{H}_{3}}}}\,\]
You need to login to perform this action.
You will be redirected in
3 sec
