Individual Members Of Monocarboxylic Acids
Category : JEE Main & Advanced
Formic Acid or Methanoic acid \[(HCOOH)\]
Formic acid is the first member of monocarboxylic acids series. It occurs in the sting of bees, wasps, red ants, stinging nettles. and fruits. In traces it is present in perspiration, urine, blood and in caterpillar's.
(1) Methods of preparation
(i) Oxidation of methyl alcohol or formaldehyde
\[C{{H}_{3}}OH+{{O}_{2}}\xrightarrow{Pt}\underset{\text{Formic acid}}{\mathop{HCOOH+{{H}_{2}}O}}\,\]
(ii) Hydrolysis of hydrocyanic acid : Formic acid is formed by the hydrolysis of HCN with acids or alkalies.
\[HCN+2{{H}_{2}}O\xrightarrow{HCl}HCOOH+N{{H}_{3}}\];
\[HCN+{{H}_{2}}O\xrightarrow{NaOH}HCOONa+N{{H}_{3}}\]
(iii) Laboratory preparation
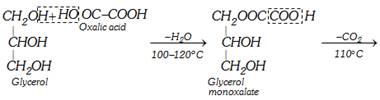
\[\underset{\begin{smallmatrix} \text{Glycerol } \\ \text{monoformate} \end{smallmatrix}}{\mathop{\underset{C{{H}_{2}}OH\,\,\,\,\,\,} {\overset{C{{H}_{2}}OOCH}{\mathop{\underset{|}{\overset{|}{\mathop{C}}}\,HOH\,\,\,\,\,\,\,\,}}}\,}}\,\xrightarrow{{{(COOH)}_{2}}2{{H}_{2}}O}\underset{\text{Formic acid}}{\mathop{HCOOH}}\,+\underset{\text{Glycerol}}{\mathop{\underset{C{{H}_{2}}OH}{\overset{C{{H}_{2}}OH}{\mathop{\underset{|}{\overset{|}{\mathop{C}}}\,HOH\,}}}\,}}\,\]
The following procedure is applied for obtaining anhydrous formic acid.
\[2HCOOH+PbC{{O}_{3}}\to \underset{\text{Lead formate}}{\mathop{{{(HCOO)}_{2}}Pb}}\,+C{{O}_{2}}+{{H}_{2}}O\];
\[{{(HCOO)}_{2}}Pb+{{H}_{2}}S\to \underset{\text{ppt}\text{.}}{\mathop{PbS}}\,+\underset{\text{Formic acid}}{\mathop{2HCOOH}}\,\]
(iv) Industrial preparation : Formic acid is prepared on industrial scale by heating sodium hydroxide with carbon monoxide at \[210{}^\circ C\] under a pressure of about 10 atmospheres.
\[CO+NaOH\underset{{{210}^{o}}C,\,10\,\text{atm}}{\mathop{\xrightarrow{\,\,\,\,\,\,\,\,\Delta \,\,\,\,\,\,\,\,}}}\,\underset{\text{Sodium formate}}{\mathop{HCOONa}}\,\]
Sodium formate thus formed is distilled with sodium hydrogen sulphate, when anhydrous formic acid distils over.
\[HCOONa+NaHS{{O}_{4}}\to HCOOH+N{{a}_{2}}S{{O}_{4}}\]
(2) Physical properties
(i) It is a colourless pungent smelling liquid.
(ii) It melts at \[8.4{}^\circ C\] and boils at \[100.5{}^\circ C\].
(iii) It is miscible with water, alcohol and ether. It forms azeotropic mixture with water.
(iv) It is strongly corrosive and cause blisters on skin.
(v) It exists in aqueous solution as a dimer involving hydrogen bonding.
(3) Uses : Formic acid is used.
(i) In the laboratory for preparation of carbon monoxide.
(ii) In the preservation of fruits.
(iii) In textile dyeing and finishing.
(iv) In leather tanning.
(v) As coagulating agent for rubber latex.
(vi) As an antiseptic and in the treatment of gout.
(vii) In the manufacture of plastics, water proofing compounds.
(viii) In electroplating to give proper deposit of metals.
(ix) In the preparation of nickel formate which is used as a catalyst in the hydrogenation of oils.
(x) As a reducing agent.
(xi) In the manufacture of oxalic acid.
Acetic Acid (Ethanoic Acid) \[(C{{H}_{3}}COOH)\]
Acetic acid is the oldest known fatty acid. It is the chief constituent of vinegar and hence its name (Latin acetum = vinegar)
(1) Preparation
(i) By oxidation of acetaldehyde (Laboratory-preparation)
\[C{{H}_{3}}CHO\underset{{{H}_{2}}S{{O}_{4}}(O)}{\mathop{\xrightarrow{N{{a}_{2}}C{{r}_{2}}{{O}_{7}}}}}\,C{{H}_{3}}COOH\]
(ii) By hydrolysis of methyl cyanide with acid
\[C{{H}_{3}}CN+2{{H}_{2}}O\xrightarrow{HCl}C{{H}_{3}}COOH+N{{H}_{3}}\]
(iii) By Grignard reagent
\[C{{H}_{3}}MgBr+C{{O}_{2}}\to C{{H}_{3}}-\overset{O}{\mathop{\overset{|\,|}{\mathop{C}}\,}}\,-OMgBr\xrightarrow{{{{H}_{2}}O}/{{{H}^{+}}}\;}\] \[\left( C{{H}_{3}}-\overset{O}{\mathop{\overset{|\,|}{\mathop{C}}\,}}\,-OH \right)\]
(iv) By hydrolysis of acetyl chloride, acetic anhydride or acetamide and ester
(a) \[\underset{\text{Ester}}{\mathop{C{{H}_{3}}COO{{C}_{2}}{{H}_{5}}}}\,+{{H}_{2}}O\xrightarrow{{{H}_{2}}S{{O}_{4}}\text{(conc}\text{.)}}\] \[C{{H}_{3}}COOH+{{C}_{2}}{{H}_{5}}OH\]
(b) \[\underset{\text{acetylchloride}}{\mathop{C{{H}_{3}}COCl+{{H}_{2}}O}}\,\xrightarrow{\text{dil}\text{.}\,HCl}C{{H}_{3}}COOH+HCl\]
(c) \[{{\left( C{{H}_{3}}CO \right)}_{2}}O+{{H}_{2}}O\xrightarrow{\text{dil}\text{.}\,HCl}2C{{H}_{3}}COOH\]
(v) Manufacture of acetic acid
(a) From ethyl alcohol (Quick vinegar process) : Vinegar is 6-10% aqueous solution of acetic acid. It is obtained by fermentation of liquors containing 12 to 15% ethyl alcohol. Fermentation is done by Bacterium Mycoderma aceti in presence of air at 30-35°C. The process is termed acetous fermentation.
\[\underset{\text{Ethyl alcohol}}{\mathop{C{{H}_{3}}C{{H}_{2}}OH}}\,+{{O}_{2}}\underset{\text{Bacter}\text{ia}}{\mathop{\xrightarrow{\text{Mycoderma aceti}}}}\,\underset{\text{Acetic acid}}{\mathop{C{{H}_{3}}COOH}}\,+{{H}_{2}}O\]
It is a slow process and takes about 8 to 10 days for completion.
In this process, the following precautions are necessary:
Acetic acid can be obtained from vinegar with the help of lime. The calcium acetate crystallised from the solution is distilled with concentrated sulphuric acid when pure acetic acid distils over.
(b) From acetylene : Acetylene is first converted into acetaldehyde by passing through 40% sulphuric acid at 60°C in presence of 1% HgSO4 (catalyst).
\[\underset{\text{Acetyle}\text{ne}}{\mathop{CH\equiv CH}}\,+{{H}_{2}}O\underset{HgS{{O}_{4}}}{\mathop{\xrightarrow{{{H}_{2}}S{{O}_{4}}(\text{dil}.)}}}\,\underset{\text{Acetaldehyde}}{\mathop{C{{H}_{3}}CHO}}\,\]
The acetaldehyde is oxidised to acetic acid by passing a mixture of acetaldehyde vapour and air over manganous acetate at \[70{}^\circ C\].
\[2C{{H}_{3}}CHO+{{O}_{2}}\underset{70{}^\circ C}{\mathop{\xrightarrow{\text{Manganous acetate}}}}\,2C{{H}_{3}}COOH\]
\[Ca{{C}_{2}}+2{{H}_{2}}O\to Ca{{(OH)}_{2}}+{{C}_{2}}{{H}_{2}}\]
The yield is very good and the strength of acid prepared is 97%. The method is also quite cheap.
(c) By the action of CO on methyl alcohol : Methyl alcohol and carbon monoxide react together under a pressure of 30 atmospheres and \[200{}^\circ C\] in presence of a catalyst cobalt octacarbonyl, \[C{{o}_{2}}{{(CO)}_{8}}\] to form acetic acid.
\[\underset{\text{Methyl alcohol}}{\mathop{C{{H}_{3}}OH+}}\,CO\underset{30atm200{}^\circ C}{\mathop{\xrightarrow{C{{o}_{2}}{{(CO)}_{8}}}}}\,\underset{\text{Acetic acid}}{\mathop{C{{H}_{3}}COOH}}\,\]
(2) Physical properties
(i) At ordinary temperature, acetic acid is a colourless, corrosive liquid with a sharp pungent odour of vinegar. It has a sour taste.
(ii) Below \[16.5{}^\circ C,\] it solidifies as an icy mass, hence it is named glacial acetic acid.
(iii) It boils at \[118{}^\circ C\]. The high boiling point of acetic acid in comparison to alkanes, alkyl halides or alcohols of nearly same molecular masses is due to more stronger hydrogen bonding between acid molecules. This also explains dimer formation of acetic acid in vapour state.
(iv) It is miscible with water, alcohol and ether in all proportions.
(v) It is good solvent for phosphorus, sulphur, iodine and many organic compounds.
(3) Uses : It is used,
(i) As a solvent and a laboratory reagent.
(ii) As vinegar for table purpose and for manufacturing pickles.
(iii) In coagulation of rubber latex.
(iv) For making various organic compounds such as acetone, acetic anhydride, acetyl chloride, acetamide and esters.
(v) For making various useful metallic acetates, such as:
(a) Basic copper acetate which is used for making green paints.
(b) Al, Fe and Cr acetates which are used as mordants in dyeing.
(c) Lead tetra-acetate which is a good oxidising agent.
(d) Basic lead acetate which is used in the manufacture of white lead.
(e) Aluminium acetate which is used in the manufacture of water-proof fabrics.
(f) Alkali acetates which are used as diuretics.
Comparison Of Formic Acid And Acetic Acid
| Property | Formic acid | Acetic acid |
| 1. Acidic nature, (i) With electro-positive metals |
Forms salts, Hydrogen is evolved. \[HCOOH+Na\to HCOONa+\frac{1}{2}{{H}_{2}}\] |
Forms salts. Hydrogen is evolved. \[C{{H}_{3}}COOH+Na\to C{{H}_{3}}COONa+\frac{1}{2}{{H}_{2}}\] |
| (ii) With bases |
Forms salts. \[HCOOH+NaOH\to HCOONa+{{H}_{2}}O\] |
Forms salts. \[C{{H}_{3}}COOH+NaOH\to C{{H}_{3}}COONa+{{H}_{2}}O\] |
| (iii) With carbonates and bicarbonates |
Forms salts. Carbon dioxide is evolved. \[HCOOH+NaHC{{O}_{3}}\to HCOONa+{{H}_{2}}O+C{{O}_{2}}\] |
Forms salts. Carbon dioxide is evolved. \[C{{H}_{3}}COOH+NaHC{{O}_{3}}\to \] \[C{{H}_{3}}COONa+{{H}_{2}}O+C{{O}_{2}}\] |
| 2. Ester formation |
Forms esters when treated with alcohols. \[HCOOH+{{C}_{2}}{{H}_{5}}OH\to HCOO{{C}_{2}}{{H}_{5}}+{{H}_{2}}O\] |
Forms esters when treated with alcohols. \[C{{H}_{3}}COOH+{{C}_{2}}{{H}_{5}}OH\xrightarrow{{{H}_{2}}S{{O}_{4}}(conc.)}\] \[C{{H}_{3}}COO{{C}_{2}}{{H}_{5}}+{{H}_{2}}O\] |
|
3. Reaction with \[PC{{l}_{5}}\] |
Forms formyl chloride which decomposes into CO and HCl. \[HCOOH+PC{{l}_{5}}\to HCOCl(HCl+CO)+POC{{l}_{3}}+HCl\] |
Forms acetyl chloride which is a stable compound. \[C{{H}_{3}}COOH+PC{{l}_{5}}\to \] \[C{{H}_{3}}COCl+POC{{l}_{3}}+HCl\] |
| 4. Heating of ammonium salt |
Forms formamide. \[HCOON{{H}_{4}}\to HCON{{H}_{2}}+{{H}_{2}}O\] |
Forms acetamide. \[C{{H}_{3}}COON{{H}_{4}}\to C{{H}_{3}}CON{{H}_{2}}+{{H}_{2}}O\] |
| 5. Heating alone |
it decomposes into \[C{{O}_{2}}\] and \[{{H}_{2}}\] \[HCOOH\to C{{O}_{2}}+{{H}_{2}}\] |
Unaffected |
|
6. Heating with conc. \[{{H}_{2}}S{{O}_{4}}\] |
Decomposed into CO and \[{{H}_{2}}O\] \[HCOOH\underset{{{H}_{2}}S{{O}_{4}}}{\mathop{\xrightarrow{Conc.}}}\,CO+{{H}_{2}}O\] |
Unaffected |
|
7. Reaction with \[C{{l}_{2}}\] in presence of red P |
Unaffected | Forms mono, di or trichloro acetic acids. |
|
8. Action of heat on salts, (i) Calcium salt |
Forms formaldehyde. \[{{(HCOO)}_{2}}Ca\to HCHO+CaC{{O}_{3}}\] |
Forms acetone. \[{{(C{{H}_{3}}COO)}_{2}}Ca\to C{{H}_{3}}COC{{H}_{3}}+CaC{{O}_{3}}\] |
| (ii) Sodium salt |
Forms sodium oxalate. \[2HCOONa\xrightarrow{\text{heat}}\underset{COONa}{\overset{COONa}{\mathop{|\,\,\,\,\,\,\,\,\,\,\,\,\,}}}\,+{{H}_{2}}\] |
Unaffected. |
| (iii) Sodium salt with soda-lime |
Forms sodium carbonate and \[{{H}_{2}}\]. \[HCOONa+NaOH\xrightarrow{CaO}N{{a}_{2}}C{{O}_{3}}+{{H}_{2}}\] |
Forms sodium carbonate and methane. \[C{{H}_{3}}COONa+NaOH\xrightarrow{CaO}\] \[C{{H}_{4}}+N{{a}_{2}}C{{O}_{3}}\] |
| 9. Electrolysis of sodium or potassium salt | It evolves hydrogen. | It forms ethane. |
|
10. On heating with \[{{P}_{2}}{{O}_{5}}\] |
Unaffected |
Forms acetic anhydride. \[2C{{H}_{3}}COOH\xrightarrow{{{P}_{2}}{{O}_{5}}}{{(C{{H}_{3}}CO)}_{2}}O+{{H}_{2}}O\] |
|
11. Reducing nature, (i) Tollen's reagent |
Gives silver mirror or black precipitate. \[HCOOH+A{{g}_{2}}O\to 2Ag+C{{O}_{2}}+{{H}_{2}}O\] |
Unaffected. |
| (ii) Fehling's solution |
Gives red precipitate \[HCOOH+2CuO\to C{{u}_{2}}O+C{{O}_{2}}+{{H}_{2}}O\] |
Unaffected. |
| (iii) Mercuric chloride |
Forms a white ppt. which changes to greyish black. \[HgC{{l}_{2}}\to H{{g}_{2}}C{{l}_{2}}\to 2Hg\] |
Unaffected. |
|
(iv) Acidified \[KMn{{O}_{4}}\] |
Decolourises | Unaffected. |
|
12. Acid (neutral solution) \[+NaHS{{O}_{3}}+\] Sodium nitroprusside. |
Greenish blue colour. | Unaffected. |
| 13. Acid (neutral solution) + neutral ferric chloride | Red colour which changes to brown ppt. on heating. | Wine red colour. |
You need to login to perform this action.
You will be redirected in
3 sec
