Laws of chemical combination
Category : NEET
Laws of Chemical Combination
Various chemical reactions take place according to the certain laws, known as the Laws of chemical combination. These are as follows,
(1) Law of conservation of mass : It was proposed by Lavoisier and verified by Landolt. According to this law, Matter is neither created nor destroyed in the course of chemical reaction though it may change from one form to other. The total mass of materials after a chemical reaction is same as the total mass before reaction.
Example : A reaction between \[AgN{{O}_{3}}\] solution and \[KI\] solution.
\[AgN{{O}_{3}}_{(aq)}\,\,+\,\,K{{I}_{(aq)}}\,\,\xrightarrow{{}}\,\,AgI+\,NaN{{O}_{3}}_{(aq)}\] (yellow ppt.)
Mass of \[AgN{{O}_{3}}_{(aq)}\,\,+\,\,\text{Mass of }K{{I}_{(aq)}}\,\,\,=\,\,\,\text{Mass of the ppt}\text{. of }AgI\,\,+\,\,\text{Mass of }NaN{{O}_{3}}_{(aq)}\]
According to the modified statement of the law, The total sum of mass and energy of the system remains constant.
(2) Law of constant or definite proportion : It was proposed by Proust. According to this law, A pure chemical compound always contains the same elements combined together in the fixed ratio of their weights whatever its methods of preparation may be.
Example : \[C{{O}_{2}}\] can be formed by either of the following processes:
(i) By heating \[CaC{{O}_{3}}\] : \[Ca\,C{{O}_{3}}\,\,\,\,\xrightarrow{\Delta }\,\,\,\,Ca\,O\,\,+\,\,C{{O}_{2}}\]
(ii) By heating \[NaHC{{O}_{3}}\] : \[2\,NaHC{{O}_{3}}\,\,\xrightarrow{\Delta }\,\,N{{a}_{2}}\,C{{O}_{3}}\,+\,{{H}_{2}}O\,\,+\,\,C{{O}_{2}}\]
\[C{{O}_{2}}\] is collected separately as a product of each reaction and the analysis of \[C{{O}_{2}}\] of each collection reveals that it has the combination ratio of carbon and oxygen as 12 : 32 by weight.
(3) Law of multiple proportion : It was proposed by Dalton and verified by Berzelius. According to this law, When two elements A and B combine to form more than one chemical compounds then different weights of A, which combine with a fixed weight of B, are in proportion of simple whole numbers.
Example : Nitrogen forms as many as five stable oxides. The analysis of these oxides \[({{N}_{2}}O,\,NO,\,{{N}_{2}}{{O}_{3}},\,{{N}_{2}}{{O}_{4}}\,\] and \[{{N}_{2}}{{O}_{5}})\] reveals that for 28 gm. nitrogen, the weight of oxygen that combines is in the ratio 16 : 32 : 48 : 64 : 80 i.e., 1 : 2 : 3 : 4 : 5 in \[{{N}_{2}}O,\,NO,\,\,{{N}_{2}}{{O}_{3}}\,,\,{{N}_{2}}{{O}_{4}}\] and \[{{N}_{2}}{{O}_{5}}\] respectively.
(4) Law of equivalent proportion or law of reciprocal proportion : It was proposed by Ritcher. According to this law, The weights of the two or more elements which separately react with same weight of a third element are also the weights of these elements which react with each other or in simple multiple of them.
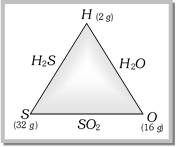
Example : Formation of \[{{H}_{2}}S,\,\,{{H}_{2}}O\] and \[S{{O}_{2}}\] can be done as follows,
(i) Hydrogen combines with sulphur forming hydrogen sulphide; 2gm. of hydrogen reacts with 32gm of sulphur. (ii) Hydrogen combines oxygen forming water; 2 gm. of hydrogen reacts with 16 gm. of oxygen. (iii) Sulphur combines with oxygen forming sulphur dioxide; 32 gm. of sulphur reacts with 32 gm. of oxygen i.e., in the ratio 32 : 32. This ratio is double of the ratio weights of these elements which combine with 2 gm. of hydrogen. i.e., 32/16 : 32/32 = 2 : 1
Law of Reciprocal proportion can be used to obtain equivalent weights of elements. As elements always combine in terms of their equivalent weights.
(5) Gay-Lussac’s Law: It was proposed by Gay–Lussac and is applicable only for gases. According to this law, When gases combine, they do so in volumes, which bear a simple ratio to each other and also to the product formed provided all gases are measured under similar conditions. The Gay-Lussac’s law, was based on experimental observation.
Example : (i) Reaction between hydrogen and oxygen. \[{{H}_{2}}_{(g)}\,\,\,+\,\,\,\frac{1}{2}\,\,{{O}_{2}}_{(g)}\,\,\,\xrightarrow{{}}\,\,{{H}_{2}}{{O}_{\,(v)}}\]
One volume of \[{{H}_{2}}\] reacts with half volume of \[{{O}_{2}}\]to give one volume of \[{{H}_{2}}\,O\].
(ii) Reaction between nitrogen and hydrogen. \[{{N}_{2}}_{(g)}\,\,+\,\,3\,{{H}_{2}}_{(g)}\,\,\,\to \,\,\,2\,N{{H}_{3}}_{(g)}\]
One volume of \[{{N}_{2}}\]reacts with three volumes of \[{{H}_{2}}\] to give two volumes of \[N{{H}_{3}}\].
Important hypothesis.
(1) Atomic hypothesis : Keeping in view various law of chemical combinations, a theoretical proof for the validity of different laws was given by John Dalton in the form of hypothesis called Dalton's atomic hypothesis. Postulates of Dalton's hypothesis is as followes,
(i) Each element is composed of extremely small particles called atoms which can take part in chemical combination.
(ii) All atoms of a given element are identical i.e., atoms of a particular element are all alike but differ from atoms of other element.
(iii) Atoms of different elements possess different properties (including different masses).
(iv) Atoms are indestructible i.e., atoms are neither created nor destroyed in chemical reactions.
(v) Atoms of elements take part to form molecules i.e., compounds are formed when atoms of more than one element combine.
(vi) In a given compound, the relative number and kinds of atoms are constant.
(2) Modern atomic hypothesis : The main modifications made in Dalton’s hypothesis as a result of new discoveries about atoms are,
(i) Atom is no longer considered to be indivisible.
(ii) Atoms of the same element may have different atomic weights. e.g., isotopes of oxygen \[{{O}^{16}},\,\,{{O}^{17}},\,\,\text{and }{{O}^{18}}\].
(iii) Atoms of different element may have same atomic weights. e.g., isobars \[C{{a}^{40}}\,\text{and }A{{r}^{40}}\].
(iv) Atom is no longer indestructible. In many nuclear reactions, a certain mass of the nucleus is converted into energy in the form of a, b and g rays.
(v) Atoms may not always combine in simple whole number ratios. e.g., in sucrose \[({{C}_{12}}{{H}_{22}}{{O}_{11}})\], the elements carbon, hydrogen and oxygen are present in the ratio of 12 : 22 : 11 and the ratio is not a simple whole number ratio.
(3) Berzelius hypothesis : “Equal volumes of all gases contain equal number of atoms under same conditions of temperature and pressure”. When applied to law of combining volumes, this hypothesis predicts that atoms are divisible and hence it is contrary to Dalton's hypothesis.
(4) Avogadro’s hypothesis : “Equal volumes of all gases under similar conditions of temperature and pressure contain equal number of molecules.” Avogadro hypothesis has been found to explain as follows:
(i) Provides a method to determine the atomic weight of gaseous elements.
(ii) Provides a relationship between vapour density (V.D.) and molecular masses of substances.
Vapour density = \[\frac{\text{Volume of definite amount of a gas}}{\text{Volume of same amount of hydrogen}}\]
or Vapour denstiy = \[\frac{Mass of 'n' molecule of a gas}{Mass of 'n' molecule of hydrogen}\]
Vapour density = \[\frac{Mass\ of\ 1\ molecule\ of\ a\ gas}{Mass\ of\ 1\ molecule\ of\ hydrogen}\]
or \[\text{Vapour density }=\text{ }\frac{Molecular mass}{2}\] or \[\text{Molecular mass }=\text{ }2\,\,\times \,\,\text{vapour density}\]
(iii) It helps in the determination of mass of fixed volume of a particular gas.
Vapour density = \[\frac{Mass of 1 molecule of gas}{Mass of 1 molecule of {{H}_{2}}}\]= \[\frac{Mass of 1 ml of gas}{Mass of 1 ml of {{H}_{2}}}\]= \[\frac{Mass of 1 ml of gas}{0.0000897}\]
(\[\because \] \[1\ ml\] \[{{H}_{2}}=0.0000897\] gm.) at NTP
\[\therefore \] Mass of \[1\ ml\] gas = V.D. ´ 0.0000897 gm.
(iv) It also helps in the determination of molar volume at N.T.P.
\[\because \] V.D. ´ 0.0000897 gm. gas has volume = \[1\ ml\]
\[\therefore \] 2 ´ V.D.(i.e., molecular mass) gm. has volume = \[\frac{1\ \times \ 2\ \times \ V.D.}{V.D.\ \times \ 0.0000897}\ ml\]= 22400\[ml\]
\[\therefore \] Molar mass of a gas or its 1 mole occupies 22.4 L volume at S.T.P.
![]()
22.4 litres of any gas at S.T.P. weigh equal to the molecular weight of the gas expressed in grams. This is called Gram-molecular volume (G.M.V.) law.
(v) It helps in determination of molecular formulae of gases and is very useful in gas analysis. By knowing the molecular volumes of reactants and products of reaction, molecular composition can be determined easily.
You need to login to perform this action.
You will be redirected in
3 sec
