The mole concept
Category : NEET
The Mole Concept
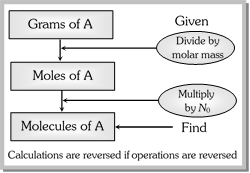
The mole (abbreviated as mol) is the SI base unit for a amount of a chemical species. It is always associated with a chemical formula and refers to Avogadro’s number (\[6.022\times {{10}^{23}}\]) of particles represented by the formula. It is designated as \[{{N}_{0}}\]. Thus, 12 molecules of \[HCl\] is a dozen, 144 molecules of \[HCl\] is a gross and \[6.022\times {{10}^{23}}\] molecules of \[HCl\] is a mole.
1 mole of a substance = \[6.022\times {{10}^{23}}\] species
The molar mass of a substance is the mass in grams of 1 mole of that substance.
\[\text{Mole of a substance }=\frac{\text{mass in grams}}{\text{molar mass}}\]
Under STP conditions when temperature is 273K and pressure is 1 atm, volume of one mole of an ideal gas is 22.4L
![]()
Example: 7 The number of gram molecules of oxygen in \[6.02\times {{10}^{24}}CO\] molecules is [IIT 1990]
(a) 10 gm molecules (b) 5 gm molecules (c) 1 gm molecules (d) 0.5 gm molecules
Solution: (b) \[6.02\times {{10}^{23}}\] molecules = 1 mole of \[CO\]
\[\therefore \] \[6.02\times {{10}^{24}}\] CO molecules = 10 moles of CO
= 10 gms of Oxygen atom =5 gm molecules of \[{{O}_{2}}\]
Example: 8 1 c.c of \[{{N}_{2}}O\] at NTP contains : [CBSE PMT 1988]
(a) \[\frac{1.8}{224}\times {{10}^{22}}\] atoms (b) \[\frac{6.02}{22400}\times {{10}^{23}}\] molecules (c) \[\frac{1.32}{224}\times {{10}^{23}}\] electrons (d) All the above
Solution: (d) 22400 c.c. = \[6.02\times {{10}^{23}}\] molecules
1 c.c. of \[{{N}_{2}}O=\frac{6.02\times {{10}^{23}}}{22400}\] molecules
\[=\frac{3\times 6.02\times {{10}^{23}}}{22400}\] atoms (Since \[{{N}_{2}}O\] has three atoms)
\[=\frac{6.02\times {{10}^{23}}}{22400}\times 22\] electron (Because number of electrons in \[{{N}_{2}}O\] are 22)
Example: 9 The mass of carbon present in 0.5 mole of \[{{K}_{4}}[Fe{{(CN)}_{6}}]\] is
(a) \[1.8\ g\] (b) \[18\ g\] (c) \[3.6\ g\] (d) \[36\ g\]
Solution: (d) 1 mole of \[{{K}_{4}}[Fe{{(CN)}_{6}}]\]=\[6\ gm\] atoms of carbon
\[0.5\] mole of \[{{K}_{4}}[Fe{{(CN)}_{6}}]\]\[=\ 3\ gm\] atoms of carbon
\[=3\times 12=36\ g\]
Example: 10 The number of moles of oxygen in one litre of air containing 21% oxygen by volume under standard conditions is [CBSE PMT 1995]
(a) 0.186 mole (b) 0.21 mole (c) 0.0093 mole (d) 2.10 mole
Solution: (c) \[\because \] 100 ml of air at STP contains 21 ml of \[{{O}_{2.}}\]
\[\therefore \] 1000 ml of air at STP contains 210 ml of \[{{O}_{2.}}\]
\[\therefore \] No. of moles of \[{{O}_{2}}\] = \[\frac{\text{Vol}\text{. of }{{O}_{2}}\,\text{in litres under}\,\text{ STP conditions }}{22.4\,\text{litre}}\] =\[\frac{210/1000}{22.4}\,=\,\frac{21}{2240}\] = 0.0093 mole
Example: 11 The number of moles of \[BaC{{O}_{3}}\,\]which contains 1.5 moles of oxygen atoms is [EAMCET 1991]
(a) 0.5 (b) 1 (c) 3 (d) 6.02 \[\times \]1023
Solution: (a) \[\because \] 1 mole of \[BaC{{O}_{3}}\]contains 3 moles of oxygen atoms.
\[\therefore \]\[\frac{1}{2}\] mole (0.5) of \[BaC{{O}_{3}}\] contains 1.5 moles of oxygen atoms.
You need to login to perform this action.
You will be redirected in
3 sec
