-
question_answer1) Which of the following solutions are isotonic with one another?
A)
0.15 M urea and 0.05 M \[1.22f\]
done
clear
B)
0.15M urea and 0.1 M\[f\]
done
clear
C)
0.15 M urea and 0.15 M glucose
done
clear
D)
0.1\[1.27f\] and 0.05 M \[\text{1}.0\text{1}\times \text{1}{{0}^{\text{5}}}\text{ N}/{{\text{m}}^{\text{2}}}\]
done
clear
View Answer play_arrow
-
question_answer2)
Five moles of a gas is put through a series of changes as shown graphically in a cyclic process. The process during \[\text{9}.\text{13}\times \text{1}{{0}^{\text{4}}}\text{ N}/{{\text{m}}^{\text{2}}}\] and \[\text{9}.\text{13}\times \text{1}{{0}^{\text{3}}}\text{N}/{{\text{m}}^{\text{2}}}\] respectively are 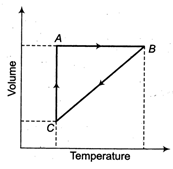
A)
isochoric, isobaric, isothermal
done
clear
B)
isobaricJsochoric, isothermal
done
clear
C)
isothermal, isobaric, isochoric
done
clear
D)
isochoric, isothermal, isobaric
done
clear
View Answer play_arrow
-
question_answer3) For the hypothetical reactions, the equilibrium constant (K) values are given \[AB,{{K}_{1}},=2.0\] \[BC,{{K}_{2}}=4.0\] \[CD;{{K}_{3}}=3.0\] The equilibrium constant (K), for the reaction \[9.13\times {{10}^{3}}N/{{m}^{2}}\]is
A)
48
done
clear
B)
6
done
clear
C)
2.7
done
clear
D)
24
done
clear
View Answer play_arrow
-
question_answer4) Standard electrode potential of half-cell reactions are given below \[C{{u}^{2+}}+2{{e}^{-}}\xrightarrow{{}}Cu;\]\[{{E}^{o}}=0.34V\] \[Z{{n}^{2+}}+2{{e}^{-}}\xrightarrow{{}}Zn;\]\[{{E}^{o}}=-0.76V\]What is the emf of the cell?
A)
\[\left[ \text{F}{{\text{L}}^{\text{2}}}{{T}^{-\text{2}}} \right]\]
done
clear
B)
\[\left[ \text{F}{{\text{L}}^{-\text{1}}}{{\text{T}}^{\text{2}}} \right]\]
done
clear
C)
\[\left[ {{\text{F}}^{2}}\text{L}{{\text{T}}^{\text{-2}}} \right]\]
done
clear
D)
\[-\text{273}.\text{15}{}^\circ \text{F}\]
done
clear
View Answer play_arrow
-
question_answer5) The product of reaction between aniline acetic anhydride is
A)
o-aminoacetophenone
done
clear
B)
m-aminoacetophenone
done
clear
C)
p-aminoacetophenone
done
clear
D)
acetanilide
done
clear
View Answer play_arrow
-
question_answer6) Anode reaction of a fuel cell is
A)
\[Zn(Hg)+2O{{H}^{-}}\xrightarrow{{}}ZnO(s)+{{H}_{2}}O+2{{e}^{-}}\]
done
clear
B)
\[Pb(s)+SO_{4}^{2-}(aq)\xrightarrow{{}}PbS{{O}_{4}}(s)+2{{e}^{-}}\]
done
clear
C)
\[2{{H}_{2}}(g)+4O{{H}^{-}}(aq)\xrightarrow{{}}4{{H}_{2}}O(l)+4{{e}^{-}}\]
done
clear
D)
done
clear
View Answer play_arrow
-
question_answer7) 2.0 g of benzoic acid dissolved in 25.0 g benzene shows a depression in freezing poi equal to 1.62 K. Molal depression constant \[\text{Vc}=\text{1}.\text{5V}\] benzene is\[\text{1}00\text{ }\mu \text{A}\]. The percenta association of the acid is
A)
80%
done
clear
B)
99%
done
clear
C)
75%
done
clear
D)
100% \[1.5\mu \] \[\mu \] Normal molecular weight of \[W\] \[\frac{4W}{3}\]=122 \[\frac{5W}{2}\] \[\frac{\pi }{2}\] \[\sigma =\text{5}.\text{67}\times \text{1}{{0}^{-\text{8}}}\text{W}-{{\text{m}}^{\text{2}}}{{\text{K}}^{\text{-4}}}\] \[2{{C}_{6}}{{H}_{5}}COOH\xrightarrow{{}}{{({{C}_{6}}{{H}_{5}}COOH)}_{2}}\] \[t\] where, \[{{(Kg)}^{1/2}}\] \[{{(Kg)}^{-1/2}}\] \[{{(Kg)}^{2}}\] % association \[{{(Kg)}^{-2}}\] \[\frac{pV}{nT}\]
done
clear
View Answer play_arrow
-
question_answer8) The number of chloride ion/s produced complex tetraminedichloroplatinum chloride in a aqueous solution is
A)
four
done
clear
B)
two
done
clear
C)
one
done
clear
D)
three
done
clear
View Answer play_arrow
-
question_answer9) When \[\text{15}0\text{ }\mu \text{A}\]is added to phenol
A)
no reaction occurs
done
clear
B)
a coloured complex will be formed
done
clear
C)
\[\text{5 mA}\]will be oxidised to higher state
done
clear
D)
o-chlprophenol will be formed
done
clear
View Answer play_arrow
-
question_answer10) \[\text{10 mA}\]on treatment with \[\text{ }\!\!\beta\!\!\text{ }\]in aqueous medium gives
A)
no reaction
done
clear
B)
\[\left( \frac{1}{V(volume)} \right)\]
done
clear
C)
\[\frac{3}{4}\text{m}/\text{s}\]
done
clear
D)
isobutylene
done
clear
View Answer play_arrow
-
question_answer11) Conduction in a p-type semiconductor is increased by
A)
increasing the band gap
done
clear
B)
decreasing the temperature
done
clear
C)
adding appropriate electron deficient impurities
done
clear
D)
adding appropriate electron rich impurities
done
clear
View Answer play_arrow
-
question_answer12) \[\frac{1}{3}\text{m}/\text{s}\] and freons
A)
are green compounds because they are green coloured
done
clear
B)
deplete ozone
done
clear
C)
cause increase in ozone concentration
done
clear
D)
have no effect on ozone concentration
done
clear
View Answer play_arrow
-
question_answer13) Boron is unable to form\[BF_{6}^{3-}\] because of
A)
high electronegativity of boron
done
clear
B)
high electronegativity of fluorine
done
clear
C)
lack of d-orbitals in boron
done
clear
D)
less difference in electronegativity between B and F
done
clear
View Answer play_arrow
-
question_answer14) The blue colour obtained in the Lassaigne test is due to formation of the compound !m
A)
\[\frac{2}{3}\text{m}/\text{s}\]
done
clear
B)
\[{{\lambda }_{0}},\]
done
clear
C)
\[\frac{25}{16}{{\lambda }_{0}}\]
done
clear
D)
\[\frac{27}{20}{{\lambda }_{0}}\]
done
clear
View Answer play_arrow
-
question_answer15) A radioactive substance decays 20% in 10 min If at the start there are \[\frac{20}{27}{{\lambda }_{0}}\] atoms present after what time will the number of atoms be reduced to \[\frac{16}{25}{{\lambda }_{0}}\] atoms?
A)
5.65 h
done
clear
B)
4.65h
done
clear
C)
3.65 h
done
clear
D)
6.65 h
done
clear
View Answer play_arrow
-
question_answer16) \[3\Omega \]of gelatin is required to be added \[4\Omega \]of a standard gold solution to just prevent its precipitation by the addition \[4.5\Omega \]of 10% \[NaCl\] solution to it. Hence, the gold number of gelatin in milligram is
A)
\[5\Omega \]
done
clear
B)
\[\frac{\sqrt{3}}{1}\]
done
clear
C)
\[\frac{(\sqrt{3}+1)}{(\sqrt{3}-1)}\]
done
clear
D)
\[\frac{(\sqrt{3}+1)}{1}\]
done
clear
View Answer play_arrow
-
question_answer17) Which of the following are arranged in the decreasing order of dipole moment?
A)
\[\frac{4}{3}\]
done
clear
B)
\[4\mu F\]
done
clear
C)
\[10\mu F\]
done
clear
D)
\[8\mu F\]
done
clear
View Answer play_arrow
-
question_answer18) If a saturated solution prepared by dissolving \[120\mu F\]in water has \[\omega \] What is the value of \[R/2\] for\[\frac{4\omega }{5}\]?
A)
\[\frac{2\omega }{5}\]
done
clear
B)
\[\frac{3\omega }{5}\]
done
clear
C)
\[93.8\times {{10}^{-12}}\]
done
clear
D)
\[9.38\times {{10}^{-12}}\]
done
clear
View Answer play_arrow
-
question_answer19) Which one of the following molecules is achiral?
A)
1-bromo-2-butene
done
clear
B)
3-bromo-l-butene
done
clear
C)
2, 3-dihydroxy propanal
done
clear
D)
2-hydroxypropanoic acid
done
clear
View Answer play_arrow
-
question_answer20)
Consider the parallel reactions in the electrophilic addition of \[\mu =\frac{4}{3}\]to propene \[{{\sin }^{-1}}\left( \frac{9}{8} \right)\] the alternative pathways shown below 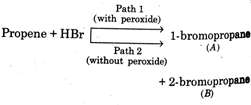 Identify the wrong statement with reference to Bafeabove
Identify the wrong statement with reference to Bafeabove
A)
Path1 has Predominance of (A) over (B)
done
clear
B)
Path-2 has predominance of (B) over (A)
done
clear
C)
Path-1 is in accordance with anti Markownikoff's rule
done
clear
D)
Both the paths afford 50% yield of (A) and (B)
done
clear
View Answer play_arrow
-
question_answer21) The raw materials for the commercial Manufacture of DDT are
A)
chlorobenzene and chloroform
done
clear
B)
chlorobenzene and chloromethane
done
clear
C)
chlorobenzene and chloral
done
clear
D)
chlorobenzene and iodoform
done
clear
View Answer play_arrow
-
question_answer22) The gas-phase reaction of nitric oxide and bromine yields nitrosyi bromide, \[2NO(g)+B{{r}_{2}}(g)\xrightarrow{{}}2NOBr(g)\] The rate law is, rate \[{{60}^{o}}\] What is the over all reaction order?
A)
1
done
clear
B)
2
done
clear
C)
3
done
clear
D)
4
done
clear
View Answer play_arrow
-
question_answer23) Bakelite is formed by polymerisation between
A)
acrylonitrile molecules
done
clear
B)
tetrafluoroethene molecules
done
clear
C)
urea and formaldehyde molecules
done
clear
D)
phenol and formaldehyde molecules
done
clear
View Answer play_arrow
-
question_answer24) Chromatographic analysis is based on. The property of
A)
diffusion
done
clear
B)
absorption
done
clear
C)
adsorption
done
clear
D)
condensation
done
clear
View Answer play_arrow
-
question_answer25) Total number of metal atoms per unit cell in a face-centred 'cubic lattice is
A)
14
done
clear
B)
8
done
clear
C)
6
done
clear
D)
4
done
clear
View Answer play_arrow
-
question_answer26) The correct order of increasing oxidising power in the series is
A)
\[VO_{2}^{+}<C{{r}_{2}}O_{7}^{2-}<MnO_{4}^{-}\]
done
clear
B)
\[\beta =0.\text{1}\]
done
clear
C)
\[{{P}_{1}}\]
done
clear
D)
\[{{P}_{2}}\]
done
clear
View Answer play_arrow
-
question_answer27) Normal human blood sugar range is \[{{P}_{1}}\]Considering density of human blood is 1.06 kg/L, if a patient's sugar level reads 720 ppm, his/her blood sugar at that time is
A)
normal
done
clear
B)
high
done
clear
C)
low
done
clear
D)
cannot say
done
clear
View Answer play_arrow
-
question_answer28) Which of the following statements is correct?
A)
The equivalent mass of \[{{P}_{2}}\]in alkaline medium is molar mass divided by five
done
clear
B)
The equivalent mass of \[\text{2}\times \text{1}{{0}^{\text{7}}}\text{m}/\text{s}\] in strongly alkaline medium is molar mass divided by three
done
clear
C)
The equivalent mass of \[\text{2}\times \text{1}{{0}^{-2}}T\] in neutral medium is molar mass divided by two
done
clear
D)
The equivalent mass of \[\left( \frac{e}{m} \right)\]in weakly acidic medium is molar mass divided by three
done
clear
View Answer play_arrow
-
question_answer29) The spin only magnetic moment of \[\text{1}.\text{76}\times \text{1}{{0}^{\text{11}}}\text{C}/\text{kg}\](at. no. for Cr is 24) is
A)
0
done
clear
B)
1.73BM
done
clear
C)
2.83BM
done
clear
D)
4.9BM
done
clear
View Answer play_arrow
-
question_answer30)
The correct relation between the following pair of compounds is 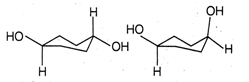
A)
constitutional isomers
done
clear
B)
enantiomers
done
clear
C)
diastereomers
done
clear
D)
None of the above
done
clear
View Answer play_arrow
-
question_answer31) The effective atomic number for\[2B\](at. no. for Rh is 45) is
A)
42
done
clear
B)
45
done
clear
C)
48
done
clear
D)
54
done
clear
View Answer play_arrow
-
question_answer32) The strongest acid among the choices is
A)
dichloroacetic acid
done
clear
B)
dimethylacetic acid
done
clear
C)
trifluoroacetic acid
done
clear
D)
triiodoacetic acid
done
clear
View Answer play_arrow
-
question_answer33) The correct order of leaving group ability in a nucleophilic substitution reaction is
A)
\[\frac{B}{4}\]
done
clear
B)
\[\frac{B}{2}\]
done
clear
C)
\[y=A\sin (Bx+Ct+D)\]
done
clear
D)
\[[{{m}^{0}}{{L}^{-1}}{{T}^{0}}]\]
done
clear
View Answer play_arrow
-
question_answer34) Glucose and fructose can be distinguished by (a) Lucas test
A)
Ninhydrin test
done
clear
B)
Benedict reagent test
done
clear
C)
All of the above
done
clear
D)
done
clear
View Answer play_arrow
-
question_answer35) Which is the most stable compound among the following?
A)
done
clear
B)
done
clear
C)
done
clear
D)
All the compounds have same stability
done
clear
View Answer play_arrow
-
question_answer36) Entropy change in a process where 1 L of liquid He is poured into ice cold water is
A)
finite and positive
done
clear
B)
finite and negative
done
clear
C)
zero
done
clear
D)
infinity
done
clear
View Answer play_arrow
-
question_answer37) For an ideal system at thermal equilibrium, the velocity distribution of the constituent particles will be governed by
A)
Gaussian distribution
done
clear
B)
Maxwell-goltzmann distribution
done
clear
C)
Lorentzian distribution
done
clear
D)
Log-normal distribution
done
clear
View Answer play_arrow
-
question_answer38) Properties of elements are periodic function of number of...... present in the nucleus.
A)
protons
done
clear
B)
electrons
done
clear
C)
neutrons
done
clear
D)
mesons
done
clear
View Answer play_arrow
-
question_answer39)
Certain reactions follow the relation between concentrations of the reactant vs time as 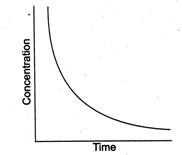 What is the expected order for such reactions?
What is the expected order for such reactions?
A)
0
done
clear
B)
1
done
clear
C)
2
done
clear
D)
Infinity
done
clear
View Answer play_arrow
-
question_answer40) Maximum number of electrons in a shell with principal quantum number n is given by
A)
\[[{{m}^{0}}{{L}^{0}}{{T}^{-1}}]\]
done
clear
B)
\[[{{m}^{0}}{{L}^{-1}}{{T}^{-2}}]\]
done
clear
C)
\[[{{m}^{0}}{{L}^{0}}{{T}^{0}}]\]
done
clear
D)
\[1.5\mu \]
done
clear
View Answer play_arrow
-
question_answer41) The first step in the extraction of Cu from copper pyrites is
A)
reduction by carbon
done
clear
B)
electrolysis of ore
done
clear
C)
roasting of ore in\[\mu \]
done
clear
D)
magnetic separation
done
clear
View Answer play_arrow
-
question_answer42) A first order reaction has a rate constant \[W\]. How long it will take to decompose half of the reaction?
A)
\[\frac{4W}{3}\]
done
clear
B)
\[\frac{5W}{2}\]
done
clear
C)
\[\frac{\pi }{2}\]
done
clear
D)
\[\sigma =\text{5}.\text{67}\times \text{1}{{0}^{-\text{8}}}\text{W}-{{\text{m}}^{\text{2}}}{{\text{K}}^{\text{-4}}}\]
done
clear
View Answer play_arrow
-
question_answer43) \[y=5\sin \frac{\pi x}{3}\cos 40\pi t\] is a
A)
strong reducing agent
done
clear
B)
strong base
done
clear
C)
strong oxidising agent
done
clear
D)
weak base
done
clear
View Answer play_arrow
-
question_answer44) A ketone gives a yellow ppt when treated with \[t\] in an alkaline solution. Thus, the ketone is
A)
a cyclic ketone
done
clear
B)
a methyl ketone
done
clear
C)
an unsaturated ketone
done
clear
D)
None of the above
done
clear
View Answer play_arrow
-
question_answer45) The ore of magnetite is
A)
\[{{(Kg)}^{1/2}}\]
done
clear
B)
\[{{(Kg)}^{-1/2}}\]
done
clear
C)
\[{{(Kg)}^{2}}\]
done
clear
D)
\[{{(Kg)}^{-2}}\]
done
clear
View Answer play_arrow
-
question_answer46) A compound with nitro group was reduced by\[\frac{pV}{nT}\], followed by treatment with \[\frac{pV}{nT}\frac{pV}{nT}\upsilon ersus\] and followed by phenol. The chromophore group in the final compound is
A)
\[{{T}_{1}}>{{T}_{2}}\] group
done
clear
B)
\[\frac{pV}{nT}\] group
done
clear
C)
\[4\times {{10}^{3}}A{{m}^{-1}}\]group
done
clear
D)
\[\text{1}{{0}^{-\text{2}}}\]group
done
clear
View Answer play_arrow
-
question_answer47) The most stable oxidation state exhibited by thallium is
A)
0
done
clear
B)
+1
done
clear
C)
+2
done
clear
D)
+3
done
clear
View Answer play_arrow
-
question_answer48) Bohr model of hydrogen atom was unable to explain
A)
Rydberg's formula of atomic spectra
done
clear
B)
Heisenberg's uncertainty principle
done
clear
C)
Planck's law of energy quantization
done
clear
D)
Rutherford's model of atomic structure
done
clear
View Answer play_arrow
-
question_answer49) The order of basic strength for methyl substituted amine in aqueous solution is
A)
\[\text{1}{{0}^{-3}}\]
done
clear
B)
\[1\mu V\]
done
clear
C)
\[\text{1}.\text{96}\times \text{1}{{0}^{-\text{8}}}\text{ m}/\text{s}\]
done
clear
D)
\[\text{2}.\text{12}\times \text{1}{{0}^{\text{8}}}\text{ m}/\text{s}\]
done
clear
View Answer play_arrow
-
question_answer50) The crystal structure of solid Mn(II) oxide is
A)
\[\text{3}.\text{18}\times \text{1}{{0}^{8}}m/s\]structure
done
clear
B)
\[\text{3}.\text{33}\times {{10}^{\text{8}}}\text{ m}/\text{s}\]structure
done
clear
C)
\[\theta =\text{45}{}^\circ \] structure
done
clear
D)
\[\frac{1}{3}M{{L}^{2}}\] structure
done
clear
View Answer play_arrow
-
question_answer51) 2-bromobutane reacts with \[\frac{3}{2}M{{L}^{2}}\]in \[\frac{3}{4}M{{L}^{2}}\] to give 2-butanol. The reaction involves
A)
retention in configuration
done
clear
B)
inversion in configuration
done
clear
C)
racemization
done
clear
D)
mutarotation
done
clear
View Answer play_arrow
-
question_answer52) The compound used for gravimetric estimation of copper \[M{{L}^{2}}\] is
A)
\[{{R}_{1}}\]
done
clear
B)
\[{{R}_{2}}\]
done
clear
C)
\[{{Q}_{1}}\]
done
clear
D)
\[{{Q}_{2}}\]
done
clear
View Answer play_arrow
-
question_answer53) Latent heat of vaporisation of water is \[{{Q}_{1}}{{R}_{2}}\ne {{Q}_{2}}{{R}_{1}}\] at\[{{Q}_{1}}{{R}_{2}}={{Q}_{2}}{{R}_{1}}\]. Calculate molal boiling point elevation constant of water.
A)
\[5.2{}^\circ \]
done
clear
B)
\[0.052{}^\circ \]
done
clear
C)
\[52.2{}^\circ \]
done
clear
D)
\[0.52{}^\circ \]
done
clear
View Answer play_arrow
-
question_answer54) The silver salt of an unknown monoacidic alkyne contains 67.08% silver. The structure of the alkyne is
A)
\[s=\frac{{{t}^{2}}}{4}\]
done
clear
B)
\[T\propto V\]
done
clear
C)
\[T\propto {{V}^{2}}\]
done
clear
D)
\[T\propto \frac{1}{{{V}^{2}}}\]
done
clear
View Answer play_arrow
-
question_answer55) Time required to deposit one millimole of As metal by the passage of 9.65A through aqueous solution of aluminium ion is
A)
30s
done
clear
B)
10s
done
clear
C)
3000s
done
clear
D)
10000s
done
clear
View Answer play_arrow

 Identify the wrong statement with reference to Bafeabove
Identify the wrong statement with reference to Bafeabove

 What is the expected order for such reactions?
What is the expected order for such reactions?
