Answer:
The
oxygen atom in both alcohols and ethers in sp3-hybridized. However,
due to greater
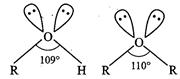 lone pair-lone pair than bond pair-bond pair repulsions, the C?O?H bond
angle in alcohols is slightly lower (109°) than the normal tetrahedral angle of
109°-28'. However, in ethers C?O?C bond angle n slightly greater (110°) than
tetrahedral angle due to repulsions between the two bulky R groups.
lone pair-lone pair than bond pair-bond pair repulsions, the C?O?H bond
angle in alcohols is slightly lower (109°) than the normal tetrahedral angle of
109°-28'. However, in ethers C?O?C bond angle n slightly greater (110°) than
tetrahedral angle due to repulsions between the two bulky R groups.
You need to login to perform this action.
You will be redirected in
3 sec
