Answer:
(i)![]() (ii)
(ii)![]() (iii) (a) p-nitroaniline<
aniline < p-toluidine.
(iii) (a) p-nitroaniline<
aniline < p-toluidine.
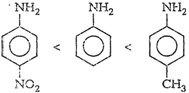 (b)
(b) ![]() (iv)
(iv)![]() (v)
(v)![]() (vi)
(vi)![]()
You need to login to perform this action.
You will be redirected in
3 sec
