Discovery of Electron
Category : 9th Class
In this chapter we will discuss about the structure of atoms and will explain the important properties of the atom and molecules. It was only in the 19th century we came to know that atom consists of sub atomic particles, called the charge of the atom. It consists of three subatomic particles, electron, proton, and neutron. The electrons are the negative charge particles, which was discovered by JJ. Thomsons in 1897.
![]() Discovery of Electron
Discovery of Electron
It was discovered by J.J. Thomson in 1897. In his experiment he took a discharge tube at very low pressure and passed electric current of high voltage through it.
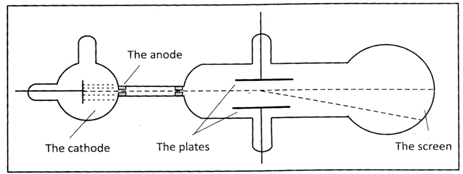
Through the gap, a small beam of cathode rays got out of the area of the cathode and anode influence. Next, the beam passed through a long vacuum tube and fell on a fluoroscopic screen leaving there a fluorescent sign. In the vacuum tube, Thomson also put two metal plates connected to a battery. That way he could create voltage between the plates, where the beam had its path. The field was directed perpendicularly to the cathode rays beam. It emerged that under the influence of voltage the beam was deflected (the spot on the screen appeared in a different place. It was the final evidence that cathode rays consisted of charged particles otherwise the beam couldn't be deflected by the electric field. The direction of the deflection also shows the charge of the particles. It emerged to of the negative charge.
It was R.A. Millikan who first measure the charge on electron in 1909, by his famous oil drop experiment. The magnitude of charge on the electron is \[\mathbf{1}{{\mathbf{0}}^{\mathbf{-19}}}\,\mathbf{coulomb}\]. The relative charge of the electron is-1. The mass of the electron is found to be
\[\mathbf{9}\mathbf{.1\times 1}{{\mathbf{0}}^{\mathbf{-31}}}\,\mathbf{kg}\]. The relative mass of an electron is approximately equal to \[\frac{1}{1840}\] of the mass of the hydrogen atom.
![]() Discovery of Proton
Discovery of Proton
The proton was discovered by E. Goldstein in 1886. In his experiment he took a discharge tube and applied high voltage into it.
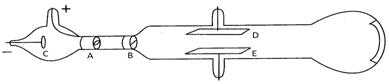
When he applied high voltage under low pressure, he observed a faint red glow on the wall behind the cathode. These rays were also called the canal rays. When these rays were allowed to pass through the charge plates placed above and below the discharge tube found that these rays were deflected towards the negative plate. He conclude that these rays must possess positive charge and hence were called protons. The charge on proton was found to be \[\mathbf{1}\mathbf{.6\times 1}{{\mathbf{0}}^{\mathbf{-19}}}\,\mathbf{coulomb}\] coulomb and the relative charge was + 1. The mass of proton was found to be \[\mathbf{1}\mathbf{.673\times 1}{{\mathbf{0}}^{\mathbf{-24}}}\,\mathbf{g}\]. The relative mass of proton was found to be 1 amu.
![]() Discovery of Neutron
Discovery of Neutron
It was discovered by James Chadwick in 1932, when he bombarded lighter elements with alpha particles and observed the emission of a highly penetrating radiation. According to him these radiation consists of neutral particles having 1amu relative mass.
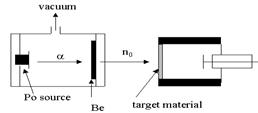
The nuclear reaction carried out during the discovery of neutrons are expressed as below:
\[^{4}B{{e}_{9}}{{+}^{2}}H{{e}_{4}}{{\to }^{6}}{{C}_{12}}{{+}^{0}}{{n}_{1}}\]

![]() A wooden log weight 25 kg. If the entire log is made up of electrons, then find the number of electrons in the wooden log.
A wooden log weight 25 kg. If the entire log is made up of electrons, then find the number of electrons in the wooden log.
(a) \[3.3\times {{10}^{31}}\]
(b) \[4.2\times {{10}^{31}}\]
(c) \[2.1\times {{10}^{31}}\]
(e) \[1.2\times {{10}^{31}}\]
(e) None of these
Answer: (a)
![]() What are cathode rays?
What are cathode rays?
(a) Positively charged
(b) Negatively charged
(c) Neutral
(d) All of these
(e) None of these
Answer: (b)
![]() Who discovered electrons?
Who discovered electrons?
(a) E. Goldestin
(b) J.J Thomsons
(c) James Chadwick
(d) Rutherford
(e) None of these
Answer: (b)
![]() In which year neutron was discovered?
In which year neutron was discovered?
(a) 1886
(b) 1897
(c) 1932
(d) 1909
(e) None of these
Answer: (c)
![]() The e/m for the proton is found to be:
The e/m for the proton is found to be:
(a) \[8.8\times {{10}^{3}}\]
(b) \[6.54\times {{10}^{3}}\]
(c) \[5.89\times {{10}^{4}}\]
(d) \[9.58\times {{10}^{4}}\]
(e) None of these
Answer: (D)
You need to login to perform this action.
You will be redirected in
3 sec
