Structure of the Atom
Category : 9th Class
Structure of the Atom
Chapter Overview
On the basis of experimental observations, different models have been proposed for the structure of an atom.
According to Thomson's model, atoms can be considered as a large sphere of uniform positive charge with a number of small negatively charged electrons scattered throughout it. This model was called as plum pudding model. The electrons present the plums in the pudding made of negative charge. This model is similar to of watermelon in which the pulp represents the positive charge and positive charge the seeds denote the electrons,
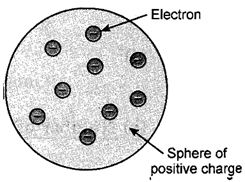
Fig. 2.1. Thomson?s plum-pudding model
(Alpha Particle Scattering Experiment)
Rutherford in 1911 performed an experiment which led to the downfall of Thomson's model According to Rutherford's model:
On the basis of the proposed model, the experimental observations in the scattering experiment. The a-particles passing through the atom in the region of the electrons would pass straight without any deflection. Only those particles that come in close vicinity of the positively charged nucleus get deviated from their path. Very few \[\alpha \]-particles, those that collide with the nucleus, would face a rebound.
In 1913 Niels Bohr, a student of Rutherford proposed a model to account for the shortcomings of Rutherford's model. Bohr's model can be understood in terms of two postulates proposed by him. The postulates are:
Postulate 1. The electrons move in definite circular paths of fixed energy around a central nucleus.
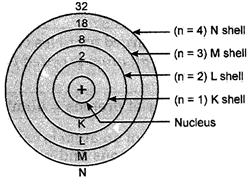
Fig. 4.1. Illustration showing different orbits or the energy levels of fixed energy in an atom according to Bohr?s model
Postulate 2. The electron can change its shells or energy level by absorbing or releasing energy. An electron at a lower state of energy \[{{E}_{i}}\] can go to a final higher state of energy \[{{E}_{f}}\]by absorbing a single photon of energy given by.
\[E=hv={{E}_{f}}-{{E}_{i}}\]
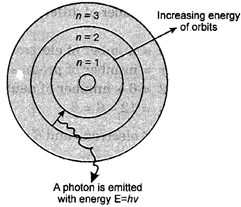
Fig. 4.2. The electrons in an atom can change their energy level by absorbing suitable amounts of energy or by emitting energy.
It was suggested that there must be one more type of subatomic particle present in the nucleus which may be neutral but have mass.
Such a particle was discovered by James Chadwick in 1932. This is found to be electrically neutral and was named neutron. Neutrons are present in the nucleus of all atoms, except hydrogen. A neutron is represented as 'n' and is found to have a mass slightly higher than that of a proton. Thus, if the helium atom contained 2 protons and 2 neutrons in the nucleus, the mass ratio of helium to hydrogen (4 : 1) could be explained. The characteristics of the three fundamental particles constituting the atom are given in Table 4.1.
Table 5.1 Characteristics of the Fundamental Subatomic Particles
|
Particle |
Symbol |
Mass (in kg) |
Actual Charge (in coulombs) |
Relative charge |
|
Electrons |
e |
\[9.109.389\times {{10}^{-31}}\] |
\[1.602177\times {{10}^{-19}}\] |
-1 |
|
Proton |
p |
\[1.672623\times {{10}^{-27}}\] |
\[1.602177\times {{10}^{-19}}\] |
1 |
|
Neutron |
n |
\[1.674928\times {{10}^{-27}}\] |
0 |
0 |
Nucleus of atom contains positively charged particles called protons and neutral particles called neutrons. The numbers of protons in an atom is called the atomic number and is denoted by the symbol "Z'.
|
Atomic number = number of protons = number of electrons |
The number of nucleons in the nucleus of an atom is called its mass number. It is denoted by 'A' and is equal to the total number of protons and neutrons present in the nucleus of an element. Thus,
|
Mass number (A) = number of protons (Z) + number of neutrons (n) |
Atomic number and mass number are represented on the symbol of an element. An element X with an atomic number, Z and the mass number, A is denoted as follows:
\[_{Z}^{A}X\]
For example, \[_{6}^{12}C\] means that the carbon has an atomic number of 6 and the mass number of 12. This can be used to compute the number of different fundamental particles in the atom.
As the atomic number is 6
\[\Rightarrow \]Number of protons = number of electrons = 6
As mass number = number of protons + number of neutrons
\[\Rightarrow \] 12 = 6 + number of neutrons
\[\Rightarrow \] number of neutrons =12 – 6=6
\[\Rightarrow \] An atom of has 6 protons, 6 electrons and 6 neutrons.
The arrangement of electrons in various shells of an atom of an element in known as electronic configuration of the element. We should know the following rules for writing down the electronic configuration of an element:
The distribution of electrons in different energy shells of an atom is governed by a schema known as Bohr-Bury Scheme. According to this scheme:
(I) These orbits or shells in an atom are represented by the letters K, L, M, N,... or the positive integral numbers, n = 1, 2, 3, 4..........
(II) The orbits are arranged in the order of increasing energy. The energy of M-shell is more than that of the L-shell which in turn is more than that of the K-shell.
(III) The maximum number of electrons present in a shell is given by the formula 2n2, where 'n' is the number of the orbit or the shell.
(IV) The shells are occupied in the increasing order of their energies.
(V) Electrons are not accommodated in a given shell, unless the inner shells are completely filled.
(VI) The outermost shell of an atom cannot accommodate more than eight electrons, even if it has the capacity to accommodate more electrons.
Table 6.1: Electronic configurations of First Twenty Elements
|
Element |
Symbol |
Atomic Number |
Electronic Distribution |
|||
|
Orbit No 1 |
2 |
3 |
4 |
|||
|
Shell K |
L |
M |
N |
|||
|
Hydrogen |
H |
1 |
1 |
|
|
|
|
Helium |
He |
2 |
2 |
|
|
|
|
Lithium |
Li |
3 |
2 |
1 |
|
|
|
Beryllium |
Be |
4 |
2 |
2 |
|
|
|
Boron |
B |
5 |
2 |
3 |
|
|
|
Carbon |
C |
6 |
2 |
4 |
|
|
|
Nitrogen |
N |
7 |
2 |
5 |
|
|
|
Oxygen |
O |
8 |
2 |
6 |
|
|
|
Fluorine |
F |
9 |
2 |
7 |
|
|
|
Neon |
Ne |
10 |
2 |
8 |
|
|
|
Sodium |
Na |
11 |
2 |
8 |
1 |
|
|
Magnesium |
Mg |
12 |
2 |
8 |
2 |
|
|
Aluminium |
Al |
13 |
2 |
8 |
3 |
|
|
Silicon |
Si |
14 |
2 |
8 |
4 |
|
|
Phosphorus |
P |
15 |
2 |
8 |
5 |
|
|
Sulphur |
S |
16 |
2 |
8 |
6 |
|
|
Chlorine |
Cl |
17 |
2 |
8 |
7 |
|
|
Argon |
Ar |
18 |
2 |
8 |
8 |
|
|
Potassium |
K |
19 |
2 |
8 |
8 |
1 |
|
Calcium |
Ca |
20 |
2 |
8 |
8 |
2 |
Valency of an element may also be defined as:
The number of hydrogen atoms or chlorine atoms or twice the number of oxygen atoms which combine with one atom of an element is called its valency.
For example, in forming water, one atom of oxygen combines with two atoms of hydrogen,
Hence, the valency of oxygen (O) in water \[({{H}_{2}}O)\] is two. The valency of aluminium in its oxide \[A{{l}_{2}}{{O}_{3}}\]is found as follows-
Number of oxygen atoms combining with two atoms of Al = 3.
Number of oxygen atoms combining with one atom of \[Al=\frac{3}{2}\]
Hence, the valency of Al (as per definition it is twice the number of oxygen atoms)
\[=2\,\times =3\]
In general, the valency of an element is equal to the number of valence electrons or is equal to eight minus the number of valence electrons.
Basically, isotope is a Greek term where isos = same and topos = place. That is, all the isotopes of an element belong to the same group and same period in the periodic table to which that element belongs.
Atoms of the some element which have same number of protons but different number of neutrons inside their nuclei are called isotopes.
Properties-1. Since the chemical properties of an element are dependent on its number of electrons, and the isotopes have same number of electrons because of same atomic number the isotopes have similar chemical properties.
when depend on the atomic mass, show variation.
Example: Isotopes of Hydrogen-There are three isotopes of hydrogen: protium, deuterium and tritium. They are represented as
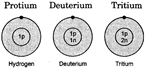
Fig. 9.1
Characteristics of Isotopes
Application of Isotopes
The atoms of different elements with different number of protons (i.e., different atomic numbers) but equal sum of the number of protons and neutrons (i.e., same mass number) are called isobars. (Greek meaning isos = equal, boros = weight), Isobars have different physical and chemical properties.
Examples:
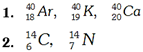
Chapter at a Glance
You need to login to perform this action.
You will be redirected in
3 sec
