Thomsons Model of Atom
Category : 9th Class
Thomson proposed the model of atom before the discovery of neutrons. According to his model, an atom consists of a positively charged sphere and electrons are embedded in it. The positive and negative charge are equal in magnitude that’s why atom is electrically neutral. His model was also known as watermelon model of an atom. Although Thomson's model was electrically neutral, but other scientist could not explain this model and hence was not accepted.
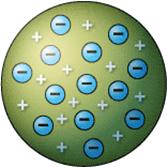
![]() Drawback of Thomsons Model
Drawback of Thomsons Model
Some of the drawbacks of Thompson's model of atom were:
You need to login to perform this action.
You will be redirected in
3 sec
