Category : 9th Class
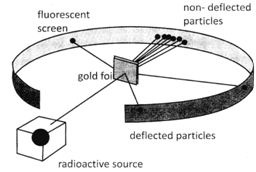
In his experiment Rutherford bombarded a thin gold foil with the fast moving alpha particles. He took a gold foil about 1000 atoms thick and a doubly charged helium ions.
![]() Observation
Observation
In his experiment Rutherford made the following observation:
![]() Conclusion
Conclusion
On the basis of his experiment and observation he made the following conclusion:
![]() Rutherford Model of Atom
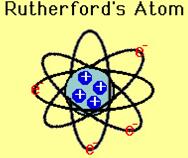
Rutherford Model of Atom
On the basis of the above experiment he proposed the following model of an atom. According to him atom consists of positively charged nucleus where the entire mass is supposed to be concentrated. The electron revolve around the nucleus in a well defined orbit and size of the nucleus is very small as compared to the size of the atom.
![]() Drawback of Rutherford Model of Atom
Drawback of Rutherford Model of Atom
Followings are the drawback of Rutherford model of an atom:
The orbital revolution of the electron is not expected to be stable. Any particle in a circular orbit would undergo acceleration. During acceleration, charged particles would radiate energy. Thus, revolving electrons would lose energy and finally fall into nucleus. If they were so, the atom would be highly unstable and hence matter would not exist in the form that we know. But this does not happen and we know that atoms are quite stable.
You need to login to perform this action.
You will be redirected in
3 sec
