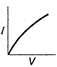question_answer 1) Which of the following rays can be polarised?
A)
Water wave and sound wave
done
clear
B)
Sound wave and radio wave
done
clear
C)
X-rays and water wave
done
clear
D)
Light wave and X-ray
done
clear
View Answer play_arrow
question_answer 2) Quantum nature can prove :
A)
Interference
done
clear
B)
photoelectric effect
done
clear
C)
diffraction
done
clear
D)
polarization
done
clear
View Answer play_arrow
question_answer 3) Which one has highest binding energy per nucleon?
A)
\[F{{e}^{56}}\]
done
clear
B)
\[L{{i}^{6}}\]
done
clear
C)
\[{{U}^{235}}\]
done
clear
D)
\[C{{a}^{40}}\]
done
clear
View Answer play_arrow
question_answer 4) Huygens wave theory cant explain:
A)
interference
done
clear
B)
photoelectric-effect
done
clear
C)
diffraction
done
clear
D)
all of these
done
clear
View Answer play_arrow
question_answer 5) If the refractive index of a glass prism is cot (A/2) and A is angle of prism, then angle of minimum deviation is:
A)
\[\left( \frac{\pi }{2}-A \right)\]
done
clear
B)
\[\left( 2\pi -\frac{A}{2} \right)\]
done
clear
C)
\[\left( \frac{\pi -A}{2} \right)\]
done
clear
D)
\[(\pi -2A)\]
done
clear
View Answer play_arrow
question_answer 6)
If\[f\] is the frequency when mass m is attached to a spring of spring constant\[k,\]then new frequency for this arrangement, is:
A)
\[f/2\]
done
clear
B)
\[\sqrt{2f}\]
done
clear
C)
\[f/\sqrt{2}\]
done
clear
D)
\[2\sqrt{2f}\]
done
clear
View Answer play_arrow
question_answer 7)
What is equivalent capacitance of the network? Each capacitor has \[\text{1}\,\text{ }\!\!\mu\!\!\text{ F}\]capacitance:
A)
\[\frac{1}{3}\]
done
clear
B)
\[2\mu F\]
done
clear
C)
\[\frac{3}{2}\mu F\]
done
clear
D)
\[3\mu F\]
done
clear
View Answer play_arrow
question_answer 8) The two capacitors \[{{\text{C}}_{\text{1}}}\]and \[{{\text{C}}_{\text{2}}}\]are charged to potentials\[{{\text{V}}_{1}}\]and\[{{\text{V}}_{2}}\] and then connected in parallel. There will be no flow of energy, if:
A)
\[{{C}_{1}}{{V}_{1}}={{C}_{2}}{{V}_{2}}\]
done
clear
B)
\[{{V}_{1}}={{V}_{2}}\]
done
clear
C)
\[{{C}_{1}}=C{{ }_{2}}\]
done
clear
D)
\[\frac{{{C}_{1}}}{{{V}_{1}}}=\frac{{{C}_{2}}}{{{V}_{2}}}\]
done
clear
View Answer play_arrow
question_answer 9) Which is not the unit of electric field?
A)
\[\frac{N}{C}\]
done
clear
B)
\[\frac{N-m}{C}\]
done
clear
C)
\[\frac{V}{m}\]
done
clear
D)
\[\frac{J}{C-m}\]
done
clear
View Answer play_arrow
question_answer 10) If a body moves for 2 s with 15 m/s velocity towards east and then moves with 5 m/s velocity for 8 s towards north, then average velocity is :
A)
5 m/s
done
clear
B)
15 m/s
done
clear
C)
30 m/s
done
clear
D)
7.5 m/s
done
clear
View Answer play_arrow
question_answer 11) For monoatomic gas which is correct?
A)
\[{{C}_{V}}=\frac{3}{5}R\]
done
clear
B)
\[{{C}_{P}}=\frac{5}{2}R\]
done
clear
C)
\[{{C}_{P}}-{{C}_{V}}=2R\]
done
clear
D)
\[\frac{{{C}_{P}}}{{{C}_{V}}}=\frac{3}{5}\]
done
clear
View Answer play_arrow
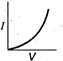
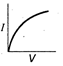
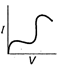
question_answer 12) Which is correct relationship for diode?
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 13) In a properly biased transistor:
A)
both depletion layers are equally large
done
clear
B)
both depletion layers are equally small
done
clear
C)
emitter-base depletion layer is large but base-collector depletion layer is small
done
clear
D)
emitter-base depletion layer is small but base-collector depletion layer is large
done
clear
View Answer play_arrow
question_answer 14) A wire has resistance \[20\,\Omega .\] If its length is increased three times its initial length, then new resistance is:
A)
\[40\,\Omega .\]
done
clear
B)
\[80\,\Omega .\]
done
clear
C)
\[60\,\Omega .\]
done
clear
D)
\[180\,\Omega .\]
done
clear
View Answer play_arrow
question_answer 15) A circular coil of diameter d is rotated in electric field such that electric flux is changed from zero to maximum value \[\text{o }\!\!|\!\!\text{ }\] then, electric field is:
A)
\[\frac{\text{o }\!\!|\!\!\text{ }}{\pi {{d}^{2}}}\]
done
clear
B)
\[\frac{\text{2o }\!\!|\!\!\text{ }}{\pi {{d}^{\,2}}}\]
done
clear
C)
\[\frac{4{{d}^{2}}}{\pi \text{o}{{\text{ }\!\!|\!\!\text{ }}^{\,2}}}\]
done
clear
D)
\[\frac{4\text{o }\!\!|\!\!\text{ }}{\pi {{d}^{2}}}\]
done
clear
View Answer play_arrow
question_answer 16) Work done in rotating a bar magnet from 0 to angle \[\text{ }\!\!\theta\!\!\text{ }\] is:
A)
\[MH(1-cos\theta )\]
done
clear
B)
\[\frac{M}{H}(1-cos\theta )\]
done
clear
C)
\[\frac{M}{H}(cos\theta -1)\]
done
clear
D)
\[MH(cos\theta -1)\]
done
clear
View Answer play_arrow
question_answer 17) If a convex lens of refractive index 1.44 is dipped in liquid of refractive index 1.49, then it behaves as:
A)
concave lens
done
clear
B)
convex lens
done
clear
C)
mirror
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 18) If a source approaches and recedes from observer with same velocity, the ratio of frequencies (apparent) is 6 : 5, then velocity of source is: \[({{v}_{s}}=330\,m/s)\]
A)
20 m/s
done
clear
B)
10 m/s
done
clear
C)
30 m/s
done
clear
D)
33 m/s
done
clear
View Answer play_arrow
question_answer 19) If a boy swings in a circle so the minimum and maximum height from ground is 3 m and 6 m, then, its maximum velocity is:
A)
\[5\sqrt{2}\,m/s\]
done
clear
B)
\[2\sqrt{5}\,m/s\]
done
clear
C)
\[3\sqrt{5}\,m/s\]
done
clear
D)
\[5\sqrt{3}\,m/s\]
done
clear
View Answer play_arrow
question_answer 20) If percentage decrease in radius of earth is 1% without changing its mass, then percentage change in acceleration due to gravity is:
A)
2% decrease
done
clear
B)
2% increase
done
clear
C)
1% decrease
done
clear
D)
1% increase
done
clear
View Answer play_arrow
question_answer 21) A black body radiates at two temperatures \[{{T}_{1}}\]and \[{{T}_{2}}\]such that \[{{T}_{1}}<{{T}_{2}}.\]The frequency corresponding to maximum intensity is:
A)
less at \[{{T}_{1}}\]
done
clear
B)
more at \[{{T}_{1}}\]
done
clear
C)
equally in the two cases
done
clear
D)
cannot say
done
clear
View Answer play_arrow
question_answer 22) If temperature is increased by 1 K at constant volume, then work done on the gas is:
A)
\[\frac{5}{2}R\]
done
clear
B)
\[\frac{3}{2}R\]
done
clear
C)
zero
done
clear
D)
\[\frac{1}{2}R\]
done
clear
View Answer play_arrow
question_answer 23) A body cools from \[\text{75}{{\,}^{\text{o}}}\text{C}\]to \[\text{70}{{\,}^{\text{o}}}\text{C}\]in time \[{{t}_{1}},\]from \[\text{70}{{\,}^{\text{o}}}\text{C}\]to \[65{{\,}^{o}}C\]in time\[{{t}_{2}}\]and from \[65{{\,}^{o}}C\]to \[60{{\,}^{o}}C\]in time \[{{t}_{3}},\] then:
A)
\[{{t}_{3}}>{{t}_{2}}>{{t}_{1}}\]
done
clear
B)
\[{{t}_{1}}>{{t}_{2}}>{{t}_{3}}\]
done
clear
C)
\[{{t}_{2}}>{{t}_{1}}={{t}_{3}}\]
done
clear
D)
\[{{t}_{1}}>{{t}_{2}}>{{t}_{3}}\]
done
clear
View Answer play_arrow
question_answer 24) A gas is at\[\text{2}{{\text{7}}^{\text{o}}}\text{C}\text{.}\] Its volume is doubled keeping pressure constant, then final temperature is:
A)
\[600{{\,}^{o}}C\]
done
clear
B)
327 K
done
clear
C)
\[327{{\,}^{o}}C\]
done
clear
D)
\[~273{{\,}^{o}}C\]
done
clear
View Answer play_arrow
question_answer 25) If the volume of gas is changed from \[{{V}_{1}}\]to \[{{V}_{2}}\]isothermally, then work done is:
A)
\[RT\,\ln \frac{{{V}_{1}}}{{{V}_{2}}}\]
done
clear
B)
\[RT\,\ln \frac{{{V}_{2}}}{{{V}_{1}}}\]
done
clear
C)
\[R({{T}_{2}}-{{T}_{1}})ln\frac{{{V}_{2}}}{{{V}_{1}}}\]
done
clear
D)
\[R({{V}_{2}}-{{V}_{1}})ln\frac{{{T}_{2}}}{{{T}_{1}}}\]
done
clear
View Answer play_arrow
question_answer 26) If energy is supplied to a gas isochorically, increase in internal energy is \[dU\]then:
A)
\[dQ=dU+dW\]
done
clear
B)
\[dQ=dU-dW\]
done
clear
C)
\[dQ=dU\]
done
clear
D)
\[dQ=-dU\]
done
clear
View Answer play_arrow
question_answer 27) A nucleus \[_{\text{Z}}^{\text{A}}\text{X}\]emits one \[\alpha \]and \[2\beta \] particles, then final nucleus is:
A)
\[Y_{Z}^{A}-2\]
done
clear
B)
\[Y_{Z-4}^{A-4}\]
done
clear
C)
\[Y_{Z}^{A-4}\]
done
clear
D)
\[X_{Z}^{A}\]
done
clear
View Answer play_arrow
question_answer 28) The fringe width for red light is approximately how many times that for violet light in Youngs slit experiment?
A)
2 times
done
clear
B)
3 times
done
clear
C)
Equal
done
clear
D)
1/2 times
done
clear
View Answer play_arrow
question_answer 29) A person sees clearly at a distance of 100 cm, then power of lens used to see object at 40 cm is:
A)
3D
done
clear
B)
\[-3D\]
done
clear
C)
\[~-1.5\text{ }D\]
done
clear
D)
\[4-1.5\text{ }D\]
done
clear
View Answer play_arrow
question_answer 30) The electric potential Vis given as a function of distance \[x\](metre) by \[V=(5{{x}^{2}}+10x-4)V.\]Value of electric field at \[x=1\,m\]is:
A)
\[-23\,V/m\]
done
clear
B)
\[11\,\,V/m\]
done
clear
C)
\[6\,V/m\]
done
clear
D)
\[-20\,V/m\]
done
clear
View Answer play_arrow
question_answer 31) In an image convenor tube fluorescent material is bombarded by:
A)
visible radiation
done
clear
B)
electron radiation
done
clear
C)
infrared radiation
done
clear
D)
ultra violet radiation
done
clear
View Answer play_arrow
question_answer 32) A particle executes, simple harmonic motion with a frequency\[f.\] The frequency with which its kinetic energy oscillates is:
A)
\[f/2\]
done
clear
B)
\[f\]
done
clear
C)
\[2f\]
done
clear
D)
\[4f\]
done
clear
View Answer play_arrow
question_answer 33) The work done by the centripetal force F when the body completes one rotation around the circle of radius R is:
A)
\[2\pi RF\]
done
clear
B)
\[~2RF\]
done
clear
C)
RF
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 34) The unit mass having \[\vec{r}=8\hat{i}-4\hat{j}\]and \[\vec{v}=8\hat{i}+4\hat{j}\]in its angular momentum is:
A)
64 unit in \[-\,\hat{k}\]direction
done
clear
B)
64 unit in \[+\,\hat{k}\]direction
done
clear
C)
64 unit in \[+\,\hat{j}\]direction
done
clear
D)
64 unit in \[-\,\,\hat{i}\] direction.
done
clear
View Answer play_arrow
question_answer 35) Which is nuclear fusion direction?
A)
Hydrogen to helium
done
clear
B)
Uranium to krypton
done
clear
C)
Hydrogen to water
done
clear
D)
Neutron to proton
done
clear
View Answer play_arrow
question_answer 36) If an AC produces same heat as that produced by a steady current of 4 A, then peak value of current is:
A)
4 A
done
clear
B)
1.56 A
done
clear
C)
5.6 A
done
clear
D)
1.41 A
done
clear
View Answer play_arrow
question_answer 37) In LCR circuit\[f=\frac{50}{\pi }Hz,\,V=50\,\text{volt,}\]\[\,\text{R}\,\text{=}\,\text{300}\,\Omega .\] If\[\text{L}\,\text{=}\,\text{1}\,\text{H}\]and \[C=20\,\mu C,\]then voltage across capacitor is:
A)
zero
done
clear
B)
20 V
done
clear
C)
30 V
done
clear
D)
50 V
done
clear
View Answer play_arrow
question_answer 38) If two forces each of 2 N are inclined at \[\text{6}{{\text{0}}^{\text{o}}}\text{,}\] then resultant force is:
A)
2N
done
clear
B)
\[2\sqrt{5}\,N\]
done
clear
C)
\[3\sqrt{2}\,N\]
done
clear
D)
\[4\sqrt{2}\,N\]
done
clear
View Answer play_arrow
question_answer 39) A block of mass 10 kg is placed on a rough horizontal surface whose coefficient of friction is 0.5. If a horizontal force of 100 N is applied on it, then acceleration of block will be:
A)
\[10\text{ }m/{{s}^{2}}\]
done
clear
B)
\[5\,m/{{s}^{2}}\]
done
clear
C)
\[15\text{ }m/{{s}^{2}}\]
done
clear
D)
\[~0.5\,m/{{s}^{2}}\]
done
clear
View Answer play_arrow
question_answer 40) The potential difference across an instrument in an AC circuit of frequency \[f\] is V and the current through it is \[I\] such, that \[V=5\cos \,2\pi ft\,\text{volt}\] and\[I=2\sin 2\pi ft\] amp. The power dissipated in the instrument is:
A)
zero
done
clear
B)
10 W
done
clear
C)
5 W
done
clear
D)
2.5 W
done
clear
View Answer play_arrow
question_answer 41) If ratio of intensities of interfering waves is 16: 9, then ratio of maximum to minimum intensity is:
A)
49 : 1
done
clear
B)
225 : 81
done
clear
C)
3:1
done
clear
D)
9:1
done
clear
View Answer play_arrow
question_answer 42) The power of a lens, a short sighted person uses is -2 D. Find the maximum distance of an object which he can see without spectacles:
A)
25 cm
done
clear
B)
50 cm
done
clear
C)
100 cm
done
clear
D)
10 cm
done
clear
View Answer play_arrow
question_answer 43) The first overtone frequency of a wave on string of length 2 m is 250 Hz. Then, its velocity is:
A)
1000 m/s
done
clear
B)
25 m/s
done
clear
C)
500 m/s
done
clear
D)
10 m/s
done
clear
View Answer play_arrow
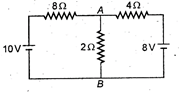
question_answer 44)
What is the current flowing in arm AB?
A)
\[\frac{35}{4}A\]
done
clear
B)
\[\frac{13}{7}A\]
done
clear
C)
\[\frac{5}{7}A\]
done
clear
D)
\[\frac{7}{5}A\]
done
clear
View Answer play_arrow
question_answer 45) A projectile is fired making an angle \[2\theta \]with horizontal with velocity 4 m/s. At any instant it makes an angle \[\theta ,\] then its velocity is:
A)
\[4\cos \theta \]
done
clear
B)
\[4(2cos\theta -sec\theta )\]
done
clear
C)
\[(2cos\theta +4sec\theta )\]
done
clear
D)
\[4(sec\theta +cos\theta )\]
done
clear
View Answer play_arrow
question_answer 46) If path difference becomes \[(2n-1)\frac{\lambda }{2}\]then: 2»
A)
white fringe is formed
done
clear
B)
bright fringe is formed
done
clear
C)
uniform illumination is obtained
done
clear
D)
dark fringe is formed
done
clear
View Answer play_arrow
question_answer 47) If the intensity of fringe at wavelength\[\lambda \] is \[K,\]then its intensity at wavelength\[\lambda /2\]is:
A)
\[\frac{K}{2}\]
done
clear
B)
\[K\]
done
clear
C)
zero
done
clear
D)
\[\sqrt{2}K\]
done
clear
View Answer play_arrow
question_answer 48) A positively charged particle moving with velocity v enters a region of space having a uniform magnetic field B. The particle will experience the large deflecting force, when the angle between v and B is:
A)
\[{{0}^{o}}\]
done
clear
B)
\[{{45}^{o}}\]
done
clear
C)
\[{{90}^{o}}\]
done
clear
D)
\[{{180}^{o}}\]
done
clear
View Answer play_arrow
question_answer 49) In a step-up transformer the turn ratio is 1:8. A lead accumulator \[(emf=6V)\] is connected across the primary coil of the .transformer. The voltage across the secondary coil is:
A)
48 V
done
clear
B)
0.75 V
done
clear
C)
14 V
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 50) The mass of a lift is 500 kg. When it ascends with an acceleration of \[\text{2}\,\text{m/}{{\text{s}}^{\text{2}}}\text{,}\] the tension in the cable will be: \[\text{(g}\,\text{=}\,\text{10}\,\text{m/}{{\text{s}}^{2}}\text{)}\]
A)
6000 N
done
clear
B)
5000 N
done
clear
C)
4000 N
done
clear
D)
1000 N
done
clear
View Answer play_arrow
question_answer 51) A water molecule can form maximum number of H-bond which is equal to:
A)
1
done
clear
B)
2
done
clear
C)
3
done
clear
D)
4
done
clear
View Answer play_arrow
question_answer 52) Bond angle in \[{{\text{H}}_{\text{2}}}\text{O}\]is:
A)
\[{{109}^{o}}28\]
done
clear
B)
\[{{107}^{o}}10\,\]
done
clear
C)
\[104.5{{\,}^{o}}\]
done
clear
D)
\[{{92}^{o}}\]
done
clear
View Answer play_arrow
question_answer 53) Calamine is:
A)
\[ZnS\]
done
clear
B)
\[PbC{{O}_{3}}\]
done
clear
C)
\[ZnC{{O}_{3}}\]
done
clear
D)
\[MgC{{O}_{3}}\]
done
clear
View Answer play_arrow
question_answer 54) Which is extremely stable?
A)
\[N{{F}_{3}}\]
done
clear
B)
\[NC{{l}_{3}}\]
done
clear
C)
\[NB{{r}_{3}}\]
done
clear
D)
\[N{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 55) \[\text{C}{{\text{H}}_{\text{3}}}\text{COCl}\]does not react with:
A)
diethyl ether
done
clear
B)
phenol
done
clear
C)
ethanol
done
clear
D)
aniline
done
clear
View Answer play_arrow
question_answer 56) In \[\text{S}{{\text{O}}_{\text{2}}}\]hybridisation is:
A)
\[sp\]
done
clear
B)
\[s{{p}^{3}}\]
done
clear
C)
\[ds{{p}^{2}}\]
done
clear
D)
\[s{{p}^{2}}\]
done
clear
View Answer play_arrow
question_answer 57) Lowest melting point chloride is:
A)
\[\text{LiCl}\]
done
clear
B)
\[\text{NaCl}\]
done
clear
C)
\[\text{KCl}\]
done
clear
D)
\[\text{CsCl}\]
done
clear
View Answer play_arrow
question_answer 58) Half-life of a substance is 6 min. If its initial amount is 32 g, then amount present after 18 minis:
A)
4g
done
clear
B)
8g
done
clear
C)
16g
done
clear
D)
2g
done
clear
View Answer play_arrow
question_answer 59) If calcium acetate and calcium formate react, then product formed is:
A)
acetaldehyde
done
clear
B)
acetic acid
done
clear
C)
formic acid
done
clear
D)
ethyl formate
done
clear
View Answer play_arrow
question_answer 60) Reduction with aluminium isopropoxide in excess of isopropyl alcohol is called Meerwein Pondorf Verley reduction (MPV). What will be the firal product when cyclohex-2-enone is selectively reduced in MPV reaction?
A)
Cyclohexanol
done
clear
B)
Cyclohex-2-enol
done
clear
C)
Cyclohexanone
done
clear
D)
Benzene
done
clear
View Answer play_arrow
question_answer 61) If pH of a solution is 4, then \[{{\text{H}}^{\text{+}}}\]is:
A)
\[\text{1}{{\text{0}}^{4}}\]
done
clear
B)
\[\text{1}{{\text{0}}^{10}}\]
done
clear
C)
\[{{10}^{-4}}\]
done
clear
D)
\[{{10}^{-10}}\]
done
clear
View Answer play_arrow
question_answer 62) When sodium nitrate is heated above \[\text{600}{{\,}^{\text{o}}}\text{C,}\]then:
A)
only \[\text{N}{{\text{a}}_{\text{2}}}\text{O}\]is formed
done
clear
B)
only \[{{\text{N}}_{2}}\]is formed
done
clear
C)
only\[{{\text{O}}_{\text{2}}}\] is formed
done
clear
D)
all are formed
done
clear
View Answer play_arrow
question_answer 63) Which of the following produces \[\text{C}{{\text{l}}_{2}}\]gas?
A)
\[\text{NaCl}\,\text{+}\,\text{HN}{{\text{O}}_{\text{3}}}\]
done
clear
B)
\[\text{Mn}{{\text{O}}_{\text{2}}}\text{+}\,\text{HCl}\]
done
clear
C)
\[\text{KMn}{{\text{O}}_{\text{4}}}\text{+}\,\text{HCl}\]
done
clear
D)
\[\text{HCl}\,\text{+}\,\text{HN}{{\text{O}}_{\text{3}}}\]
done
clear
View Answer play_arrow
question_answer 64) Which is correctly arranged as increasing size?
A)
\[F<O<C<Cl<Br\]
done
clear
B)
\[C<O<F<Cl<Br\]
done
clear
C)
\[Cl<Br<F<C<O\]
done
clear
D)
\[O<F<C<Cl<Br\]
done
clear
View Answer play_arrow
question_answer 65) \[\text{N}{{\text{H}}_{\text{3}}}\]is absorbed by:
A)
ozone
done
clear
B)
\[\text{CaO}\]
done
clear
C)
pyrargallol
done
clear
D)
\[\text{CaC}{{\text{l}}_{\text{2}}}\]
done
clear
View Answer play_arrow
question_answer 66) \[\text{1}\text{.25}\,\text{g}\,\text{N}{{\text{H}}_{\text{3}}}\]contains how many atoms?
A)
\[{{10}^{23}}\]
done
clear
B)
\[2\times {{10}^{23}}\]
done
clear
C)
\[6\times {{10}^{13}}\]
done
clear
D)
\[4\times {{10}^{23}}\]
done
clear
View Answer play_arrow
question_answer 67) Which of the following has smallest bond angle?
A)
Ethane
done
clear
B)
Ethene
done
clear
C)
Ethyne
done
clear
D)
Ethanol
done
clear
View Answer play_arrow
question_answer 68) Chloroform in air is oxidised to:
A)
\[\text{CC}{{\text{l}}_{\text{4}}}\]
done
clear
B)
dichloromethane
done
clear
C)
phosgene
done
clear
D)
oxygen
done
clear
View Answer play_arrow
question_answer 69) Gypsum is:
A)
\[CaS{{O}_{4}}.2{{H}_{2}}O\]
done
clear
B)
\[CaS{{O}_{4}}.\frac{1}{2}{{H}_{2}}O\]
done
clear
C)
\[MgS{{O}_{4}}.2{{H}_{2}}O\]
done
clear
D)
\[CuS{{O}_{4}}\]
done
clear
View Answer play_arrow
question_answer 70) Which is not soluble in water?
A)
\[PbS{{O}_{4}}\]
done
clear
B)
\[CdS{{O}_{4}}\]
done
clear
C)
\[Bi{{(S{{O}_{4}})}_{2}}\]
done
clear
D)
\[CuS{{O}_{4}}\]
done
clear
View Answer play_arrow
question_answer 71) Which of the following is colour red?
A)
\[C{{u}_{2}}O\]
done
clear
B)
\[CuF\]
done
clear
C)
\[Zn{{F}_{2}}\]
done
clear
D)
\[ZnC{{l}_{2}}\]
done
clear
View Answer play_arrow
question_answer 72) How many unpaired electrons are present in \[[Cr{{(NH)}_{3}}]B{{r}_{3}}\]
A)
1
done
clear
B)
2
done
clear
C)
3
done
clear
D)
4
done
clear
View Answer play_arrow
question_answer 73) \[S+\frac{3}{2}{{O}_{2}}\xrightarrow{{}}S{{O}_{3}}\,\Delta H=y,\] then heat of formation of \[\text{S}{{\text{O}}_{\text{2}}}\]is:
A)
\[~2x-y\]
done
clear
B)
\[~2x+y\]
done
clear
C)
\[~x+y\]
done
clear
D)
\[\frac{2x-y}{2}\]
done
clear
View Answer play_arrow
question_answer 74) Reagent (catalyst) used in Friedel-Crafts alkylation reaction is:
A)
\[AlC{{l}_{3}}\]
done
clear
B)
\[\text{anhyd}\text{.}\,\,\text{AlC}{{\text{l}}_{\text{3}}}\]
done
clear
C)
\[{{N}_{2}}\]
done
clear
D)
\[He\]
done
clear
View Answer play_arrow
question_answer 75) Catalyst used in making\[{{\text{H}}_{\text{2}}}\text{S}{{\text{O}}_{\text{4}}}\] in contact process is:
A)
\[{{\text{V}}_{\text{2}}}{{\text{O}}_{\text{5}}}\]
done
clear
B)
\[F{{e}_{2}}{{O}_{3}}\]
done
clear
C)
\[C{{r}_{2}}{{O}_{3}}\]
done
clear
D)
\[Cr{{O}_{3}}\]
done
clear
View Answer play_arrow
question_answer 76) When acetamide is reacted with \[\text{NaOBr,}\]then product formed is:
A)
ethanamine
done
clear
B)
methanamine
done
clear
C)
methanamide
done
clear
D)
ethanenitrile
done
clear
View Answer play_arrow
question_answer 77) Isocyanide is prepared by:
A)
Friedel Crafts reaction
done
clear
B)
Wurtzs reaction
done
clear
C)
Williamson synthesis
done
clear
D)
Carbylamine reaction
done
clear
View Answer play_arrow
question_answer 78) If rate of diffusion of \[\text{C}{{\text{H}}_{\text{4}}}\]is twice than that of a gas\[x,\] then its molecular mass is:
A)
64 g
done
clear
B)
16 g
done
clear
C)
32 g
done
clear
D)
8 g
done
clear
View Answer play_arrow
question_answer 79) Which one is not Lewis acid?
A)
\[Be{{F}_{2}}\]
done
clear
B)
\[SnC{{l}_{4}}\]
done
clear
C)
\[\text{AlC}{{\text{l}}_{\text{3}}}\]
done
clear
D)
\[B{{F}_{3}}\]
done
clear
View Answer play_arrow
question_answer 80) Natural gas mainly consists of:
A)
methane
done
clear
B)
butane
done
clear
C)
propane
done
clear
D)
ethane + octane
done
clear
View Answer play_arrow
question_answer 81) Phenol is treated with Zn to form:
A)
benzoic acid
done
clear
B)
benzyl alcohol
done
clear
C)
benzene
done
clear
D)
benzoquinone
done
clear
View Answer play_arrow
question_answer 82) Which one is isoelectronic with\[\text{CO}\]?
A)
\[N_{2}^{-}\]
done
clear
B)
\[N_{2}^{+}\]
done
clear
C)
\[C{{N}^{-}}\]
done
clear
D)
\[NO\]
done
clear
View Answer play_arrow
question_answer 83) How much volume of \[\text{1M}\,{{\text{H}}_{\text{2}}}\text{S}{{\text{O}}_{\text{4}}}\]is required to neutralize 20 mL of\[\text{1M}\,\text{NaOH}\]?
A)
10 mL
done
clear
B)
20 mL
done
clear
C)
5mL
done
clear
D)
15 mL
done
clear
View Answer play_arrow
question_answer 84) Fentons reagent is:
A)
\[\text{SnC}{{\text{l}}_{\text{2}}}\text{+}\,\text{HCl}\]
done
clear
B)
\[AgN{{O}_{3}}+N{{H}_{4}}OH\]
done
clear
C)
\[CuS{{O}_{4}}+NaOH\]
done
clear
D)
\[FeS{{O}_{4}}+{{H}_{2}}{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 85) \[C{{u}^{2}}+Ag\xrightarrow{{}}Cu+A{{g}^{+}}\]oxidation half reaction is:
A)
\[C{{u}^{2+}}\to Cu\]
done
clear
B)
\[Ag\to A{{g}^{+}}\]
done
clear
C)
\[Cu\to C{{u}^{2+}}\]
done
clear
D)
all of these
done
clear
View Answer play_arrow
question_answer 86) \[{{\text{C}}_{\text{4}}}{{\text{H}}_{\text{10}}}\text{O}\]has how many isomeric alcohols?
A)
1
done
clear
B)
2
done
clear
C)
3
done
clear
D)
4
done
clear
View Answer play_arrow
question_answer 87) IUPAC name of \[C{{H}_{3}}-C\equiv C-HC-{{(C{{H}_{3}})}_{2}}\] is:
A)
\[4-\]methyl\[-2-\]pentyne
done
clear
B)
\[1,1-\] dimethyl\[-2-\]butyne
done
clear
C)
\[2-\]methyl\[-4-\]pentyne
done
clear
D)
\[4,4-\]dimethyl\[-2-\]butyne
done
clear
View Answer play_arrow
question_answer 88) Fehling test is given by:
A)
glucose
done
clear
B)
fructose
done
clear
C)
sucrose
done
clear
D)
all of these
done
clear
View Answer play_arrow
question_answer 89) Number of isomeric primary amine of molecular formula \[{{\text{C}}_{\text{4}}}{{\text{H}}_{\text{11}}}\text{N}\]is:
A)
1
done
clear
B)
2
done
clear
C)
3
done
clear
D)
4
done
clear
View Answer play_arrow
question_answer 90) For reaction \[3X+Y{{X}_{3}}Y\] \[\Delta H=+\,ve,\] amount of \[{{X}_{3}}Y\]can be changed by:
A)
changing temperature
done
clear
B)
changing pressure
done
clear
C)
changing temperature, pressure,
done
clear
D)
changing temperature, pressure, adding catalyst
done
clear
View Answer play_arrow
question_answer 91) Which one is most ionic?
A)
\[{{P}_{2}}{{O}_{5}}\]
done
clear
B)
\[Mn{{O}_{2}}\]
done
clear
C)
\[M{{n}_{2}}{{O}_{7}}\]
done
clear
D)
\[{{P}_{2}}{{O}_{3}}\]
done
clear
View Answer play_arrow
question_answer 92) Which one gives \[{{I}_{2}}\] on reaction with\[KI\]?
A)
\[~A{{g}_{2}}S{{O}_{4}}\]
done
clear
B)
\[CuS{{O}_{4}}\]
done
clear
C)
\[~PbS{{O}_{4}}\]
done
clear
D)
\[~CdS{{O}_{4}}\]
done
clear
View Answer play_arrow
question_answer 93) Colour of the solution When \[\text{KI}\]reacts with \[\text{B}{{\text{r}}_{2}}\] is:
A)
blue
done
clear
B)
black
done
clear
C)
red
done
clear
D)
no change
done
clear
View Answer play_arrow
question_answer 94) Finely divided iron combines with CO to give:
A)
\[Fe{{(CO)}_{5}}\]
done
clear
B)
\[F{{e}_{2}}{{(CO)}_{9}}\]
done
clear
C)
\[F{{e}_{3}}{{(CO)}_{12}}\]
done
clear
D)
\[Fe{{(CO)}_{6}}\]
done
clear
View Answer play_arrow
question_answer 95) Which of the following is pyramidal?
A)
\[PC{{l}_{3}}\]
done
clear
B)
\[CO_{3}^{2-}\]
done
clear
C)
\[S{{O}_{2}}\]
done
clear
D)
\[NO_{3}^{-}\]
done
clear
View Answer play_arrow
question_answer 96) Which of the following conducts electricity?
A)
Crystal\[\text{NaCl}\]
done
clear
B)
Diamond
done
clear
C)
Molten\[\text{KBr}\]
done
clear
D)
Sulphur
done
clear
View Answer play_arrow
question_answer 97) Ratio of kinetic energy of hydrogen and helium gas at \[\text{300}\,\text{K}\]is:
A)
2 : 1
done
clear
B)
4 : 5
done
clear
C)
1 : 1
done
clear
D)
1 : 2
done
clear
View Answer play_arrow
question_answer 98) Which of the following has highest energy?
A)
\[n=2,\,l=1\]
done
clear
B)
\[n=3,\,l=2\]
done
clear
C)
\[n=3,\,l=1\]
done
clear
D)
\[n=2,\,l=0\]
done
clear
View Answer play_arrow
question_answer 99) Oxidation state of phosphorus in pyrophosphoric acid is:
A)
+ 5
done
clear
B)
+ 3
done
clear
C)
+ 4
done
clear
D)
+ 1
done
clear
View Answer play_arrow
question_answer 100) If the 75% of a first order reaction is complete in 8 min, then time taken to decompose 50% of its initial amount is:
A)
2 min
done
clear
B)
4 min
done
clear
C)
12 min
done
clear
D)
1 min
done
clear
View Answer play_arrow
question_answer 101) Sequence of cytochromes is:
A)
\[cyt.\text{ }a,\text{ }b,\text{ }c,\text{ }{{a}_{3}}\]
done
clear
B)
\[~cyt.\text{ }b,\text{ }c,\text{ }a,\text{ }{{a}_{3}}\]
done
clear
C)
\[~cyt.\text{ }b,\text{ }a,\text{ }{{a}_{3}},c\]
done
clear
D)
\[~cyt.\text{ }b,c,\,{{a}_{3}},a\]
done
clear
View Answer play_arrow
question_answer 102) Cytochrome is a:
A)
Mg pyrole ring
done
clear
B)
Fe porphyrin ring
done
clear
C)
nucleotide
done
clear
D)
alloy of nichrome
done
clear
View Answer play_arrow
question_answer 103) Placentation in Brasicaceae is:
A)
parietal
done
clear
B)
marginal
done
clear
C)
axile
done
clear
D)
basal
done
clear
View Answer play_arrow
question_answer 104) Rauwolffia serpentina is used in:
A)
curing high blood pressure
done
clear
B)
kideny failure
done
clear
C)
eye defect
done
clear
D)
diabetes
done
clear
View Answer play_arrow
question_answer 105) Which one of the following is agranulocyte?
A)
Neutrophil
done
clear
B)
Eosinophil
done
clear
C)
Basophil
done
clear
D)
Monocyte
done
clear
View Answer play_arrow
question_answer 106) Who described \[{{\text{C}}_{\text{4}}}\]pathway for firsts time?
A)
Hatch and Slack
done
clear
B)
Robert Hill
done
clear
C)
Hans Krebs
done
clear
D)
Melvin Calvin
done
clear
View Answer play_arrow
question_answer 107) Who is the father of Indian Bryology?
A)
O.P. lyengar
done
clear
B)
S. Kashyap
done
clear
C)
E. J. Butlar
done
clear
D)
R. Mishra
done
clear
View Answer play_arrow
question_answer 108) Which one is absent in frog?
A)
Phrenic nerve
done
clear
B)
Renal portal vein
done
clear
C)
Both [a] and [b]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 109) Poison gland in snake is located in:
A)
parietal
done
clear
B)
maxilla
done
clear
C)
mandible
done
clear
D)
neck
done
clear
View Answer play_arrow
question_answer 110) Eugene P. Odum is a:
A)
bryologist
done
clear
B)
ecologist
done
clear
C)
gerontologist
done
clear
D)
neurologist
done
clear
View Answer play_arrow
question_answer 111) Which of the following is not endocrine gland?
A)
Thyroid
done
clear
B)
Adrenal
done
clear
C)
Pituitary
done
clear
D)
Sebaceous
done
clear
View Answer play_arrow
question_answer 112) Animal protected in Kaziranga National park is:
A)
tiger
done
clear
B)
lion
done
clear
C)
elephant
done
clear
D)
Rhinoceros
done
clear
View Answer play_arrow
question_answer 113) In 125 amino acid sequence if 25 amino acid is mutated to UAA, then:
A)
a polypeptide of 124 amino acid is formed
done
clear
B)
a polypeptide of 25 amino acid is formed
done
clear
C)
a polypeptide of 24 amino acid is formed
done
clear
D)
any of the above can be possible
done
clear
View Answer play_arrow
question_answer 114) In gastrulation, which is (are) formed?
A)
endoderm
done
clear
B)
mesoderm
done
clear
C)
etoderm, endoderm
done
clear
D)
ectoderm, mesoderm, endoderm
done
clear
View Answer play_arrow
question_answer 115) In embryo sac, \[n,\text{ }2n,\text{ }3n\]conditions are found respectively in:
A)
egg, antipodal, endosperm
done
clear
B)
nucleus, endosperm, egg .
done
clear
C)
antipodal, zygote, endosperm
done
clear
D)
endosperm; nucleus, egg
done
clear
View Answer play_arrow
question_answer 116) Which of the following set is green-house gases?
A)
\[CFC,C{{H}_{4}},C{{O}_{2}},{{N}_{2}}O\]
done
clear
B)
\[C{{O}_{2}},C{{H}_{4}},{{N}_{2}},{{O}_{2}}\]
done
clear
C)
\[C{{O}_{2}},C{{H}_{4}},{{N}_{2}},{{O}_{3}}\]
done
clear
D)
\[C{{O}_{2}},CFC,{{N}_{2}},{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 117) Krebs cycle takes place in:
A)
mitochondrial matrix
done
clear
B)
cytoplasm
done
clear
C)
lysosome
done
clear
D)
nucleus
done
clear
View Answer play_arrow
question_answer 118) Which of the following is monogenetic parasite?
A)
Entamoeba histolytica
done
clear
B)
Taenia solium
done
clear
C)
Wuchereria bancroftii
done
clear
D)
Plasmodium vivax
done
clear
View Answer play_arrow
question_answer 119) Stratified squammous epithelium is found in :
A)
pharynx
done
clear
B)
trachea
done
clear
C)
-ileum
done
clear
D)
Bowmans capsule
done
clear
View Answer play_arrow
question_answer 120) Which one is polymer?
A)
Sucrose
done
clear
B)
Glycogen
done
clear
C)
Fructose
done
clear
D)
Lactose
done
clear
View Answer play_arrow
question_answer 121) In cell division, spindle fibres are made up of protein:
A)
myoglobin
done
clear
B)
tubulin
done
clear
C)
albumin
done
clear
D)
myosin
done
clear
View Answer play_arrow
question_answer 122) In photosynthesis, for fixation of one mole of glucose number of ATP and \[\text{NADP}{{\text{H}}_{\text{2}}}\]required is:
A)
12 and 18
done
clear
B)
18 and 12
done
clear
C)
6 and 12
done
clear
D)
18 and 18
done
clear
View Answer play_arrow
question_answer 123) Bulk of histone proteins synthesized in:
A)
\[{{G}_{1}}-\]phase
done
clear
B)
\[{{G}_{2}}-\] phase
done
clear
C)
S-phase
done
clear
D)
\[{{G}_{0}}-\]phase
done
clear
View Answer play_arrow
question_answer 124) Species which is in danger of extinction is:
A)
endangered
done
clear
B)
vulnerable
done
clear
C)
rare
done
clear
D)
critically endangered
done
clear
View Answer play_arrow
question_answer 125) Mollusca is:
A)
triploblastic, acoeiomate
done
clear
B)
triploblastic, coelomate
done
clear
C)
dibloblastic, acoeiomate
done
clear
D)
diploblastic, coelomate
done
clear
View Answer play_arrow
question_answer 126) Cyathium and hypanthodium inflorescence are related in having:
A)
nectar glands
done
clear
B)
unisexual flower
done
clear
C)
both [a] and [b]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 127) Exarch and polyarch condition found in:
A)
monocot stem
done
clear
B)
dicot stem
done
clear
C)
monocot root
done
clear
D)
dicot root
done
clear
View Answer play_arrow
question_answer 128) Which of the following enzymes helps in crossing plasma membrane?
A)
protease
done
clear
B)
pepsin
done
clear
C)
dehydrpgenase
done
clear
D)
permease
done
clear
View Answer play_arrow
question_answer 129) \[\text{N}{{\text{H}}_{\text{3}}}\]in Amoeba is excreted by:
A)
food vacuole
done
clear
B)
contractile vacuole
done
clear
C)
plasma membrane
done
clear
D)
all of the above
done
clear
View Answer play_arrow
question_answer 130) Antiparallel strands in DNA is due to:
A)
disulphite linkage
done
clear
B)
hydrogen bond
done
clear
C)
phosphodiester bond
done
clear
D)
glycosidic bond
done
clear
View Answer play_arrow
question_answer 131) Choose the incorrect match:
A)
nucleus-RNA
done
clear
B)
lysosome-protein synthesis
done
clear
C)
mitochondria-respiration
done
clear
D)
cytoskeleton-microtubules
done
clear
View Answer play_arrow
question_answer 132) More advancement in genetic engineering is due to:
A)
restriction endonuclease
done
clear
B)
reverse transcriptase
done
clear
C)
protease
done
clear
D)
zymase
done
clear
View Answer play_arrow
question_answer 133) Rough endoplasmic reticulum is associated with:
A)
fat synthesis
done
clear
B)
steroid synthesis
done
clear
C)
protein synthesis
done
clear
D)
all of these
done
clear
View Answer play_arrow
question_answer 134) In lung, maximum gaseous exchange is due to:
A)
simple diffusion
done
clear
B)
active transport
done
clear
C)
passive transport
done
clear
D)
fascilitated diffusion
done
clear
View Answer play_arrow
question_answer 135) Resolving power of electron microscope is:
A)
\[\text{1}\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
B)
\[\text{10}\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
C)
\[\text{100}\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
D)
\[\text{1000}\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
View Answer play_arrow
question_answer 136) The female children of haemophilic father and carrier mother:
A)
all haemophilic
done
clear
B)
half haemophilic, half carrier
done
clear
C)
all normal
done
clear
D)
all carrier
done
clear
View Answer play_arrow
question_answer 137) Number of Barr bodies in XXXXY is:
A)
1
done
clear
B)
2
done
clear
C)
3
done
clear
D)
4
done
clear
View Answer play_arrow
question_answer 138) Natural system of classification is given by:
A)
Benthem and Hooker
done
clear
B)
Carolus Linnaeus
done
clear
C)
Charles Darwin
done
clear
D)
Engler and Pranti
done
clear
View Answer play_arrow
question_answer 139) Leaves of grasses roll and unroll due to :
A)
hormonal change
done
clear
B)
change in temperature
done
clear
C)
change in turgor pressure
done
clear
D)
presence of bulliform apparatus
done
clear
View Answer play_arrow
question_answer 140) Stomata open and close due to:
A)
turgor pressure change
done
clear
B)
hormonal change
done
clear
C)
temperature change
done
clear
D)
all of the above
done
clear
View Answer play_arrow
question_answer 141) Haploid structure of Funaria is:
A)
protonema
done
clear
B)
calyptra
done
clear
C)
apophysis
done
clear
D)
operculum
done
clear
View Answer play_arrow
question_answer 142) Fruiting body of Penicillium is:
A)
cleistothecium
done
clear
B)
pycniophysis
done
clear
C)
sterigmata
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 143) Sperm of Cycas is:
A)
multiflagellated and very large
done
clear
B)
small and biflagellated
done
clear
C)
multiflagellated and small
done
clear
D)
large and biflagellated
done
clear
View Answer play_arrow
question_answer 144) Ecosystem has:
A)
plant and animal
done
clear
B)
air and \[{{\text{H}}_{\text{2}}}\text{O}\]
done
clear
C)
soil and light
done
clear
D)
biotic and abiotic component
done
clear
View Answer play_arrow
question_answer 145) For nitrogen fixation, pigment useful is :
A)
nitrogenase
done
clear
B)
haemoglobin
done
clear
C)
myoglobin
done
clear
D)
leghaemoglobin
done
clear
View Answer play_arrow
question_answer 146) Sal and teak are found in
A)
tropical rain forest
done
clear
B)
tropical deciduous forest
done
clear
C)
temperate board leaf forest
done
clear
D)
temperate needle leaf forest
done
clear
View Answer play_arrow
question_answer 147) In plasmolysed cell, the space between nucleus and plasma membrane is occupied by:
A)
hypotonic solution
done
clear
B)
hypertonic solution
done
clear
C)
isotonic solution
done
clear
D)
air
done
clear
View Answer play_arrow
question_answer 148) During oogenis, the small structure separated from egg is:
A)
polar bodies
done
clear
B)
secondary endosperm
done
clear
C)
herring bodies
done
clear
D)
Hela cells
done
clear
View Answer play_arrow
question_answer 149) Structural and functional unit of kidney is:
A)
neuron
done
clear
B)
nephron
done
clear
C)
lungs
done
clear
D)
Schwanns cells
done
clear
View Answer play_arrow
question_answer 150) Which hexaploid wheat is used to make bread?
A)
Triticum turgidum
done
clear
B)
Triticum durum
done
clear
C)
Triticum monococcum
done
clear
D)
Triticum aestivum
done
clear
View Answer play_arrow