
 INTRODUCTION
Food is the most important and basic thing for life. Carbohydrates, proteins, fats, vitamins and minerals are the components of food. These components are necessary for all living beings. All plants and animals require food for their growth and to get energy. The process of utilization of food by an animal to obtain energy for growth and development is known as nutrition, Plants make their food themselves but animals cannot. Hence, animals depend directly or indirectly on the plant.
MODE OF NUTRITION IN PLANTS
Plants obtain their nutrition by various modes. The mode of nutrition can be divided into two distinct types. Broadly speaking, plants can be divided into autotrophs and heterotrophs.
INTRODUCTION
Food is the most important and basic thing for life. Carbohydrates, proteins, fats, vitamins and minerals are the components of food. These components are necessary for all living beings. All plants and animals require food for their growth and to get energy. The process of utilization of food by an animal to obtain energy for growth and development is known as nutrition, Plants make their food themselves but animals cannot. Hence, animals depend directly or indirectly on the plant.
MODE OF NUTRITION IN PLANTS
Plants obtain their nutrition by various modes. The mode of nutrition can be divided into two distinct types. Broadly speaking, plants can be divided into autotrophs and heterotrophs.
 Autotrophic Nutrition
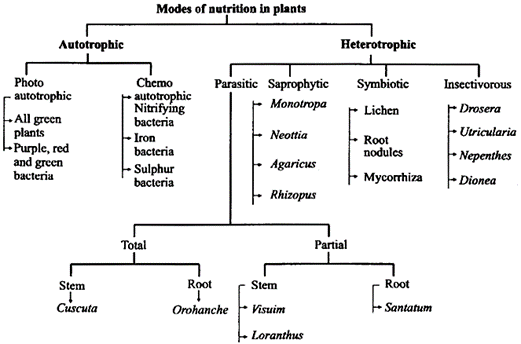
Auto means self and trophos means nourishment. Plants are called autotrophs because they make their food themselves. The making of food for themselves is called the Autotrophic nutrition. Autotrophic nutrition is found in green plants. If the autotrophs prepare their own food by utilizing chemical energy they are called chemoautotrophs.
Heterotrophic Nutrition
The word Heterotrophic is the combination of two words i.e. Hetero + Trophos.
Hetero means 'others' and 'trophos' means nourishment. If organisms depend on others for their food, it is called the Hetetrophic Nutrition. Animals cannot make their food themselves. They depend for food upon plants. Therefore, nutrition in animals is called Hetetrophic Nutrition. Animals are known as Heterotrophs.
Autotrophic Nutrition
Auto means self and trophos means nourishment. Plants are called autotrophs because they make their food themselves. The making of food for themselves is called the Autotrophic nutrition. Autotrophic nutrition is found in green plants. If the autotrophs prepare their own food by utilizing chemical energy they are called chemoautotrophs.
Heterotrophic Nutrition
The word Heterotrophic is the combination of two words i.e. Hetero + Trophos.
Hetero means 'others' and 'trophos' means nourishment. If organisms depend on others for their food, it is called the Hetetrophic Nutrition. Animals cannot make their food themselves. They depend for food upon plants. Therefore, nutrition in animals is called Hetetrophic Nutrition. Animals are known as Heterotrophs.
 Heterotrophic plants can be further divided into parasites, saprophytes and symbiotic plants.
Heterotrophic plants can be further divided into parasites, saprophytes and symbiotic plants.
 Photosynthesis - Food Making Process in Plants
The process of making of food by green plants in the presence of sunlight and chlorophyll is known as photosynthesis. Photosynthesis is the combination of two words- Photo + Synthesis. 'Photo' means light and 'Synthesis' means to make.
Process of Food Making in Green Plants
Green plants make their food themselves. Green leaves make food from Carbon dioxide and water in the presence of sunlight and chlorophyll. Hence, for taking place of photosynthesis carbon dioxide, water and sunlight must be reached at the green leaves in addition to presence of chlorophyll.
Photosynthesis - Food Making Process in Plants
The process of making of food by green plants in the presence of sunlight and chlorophyll is known as photosynthesis. Photosynthesis is the combination of two words- Photo + Synthesis. 'Photo' means light and 'Synthesis' means to make.
Process of Food Making in Green Plants
Green plants make their food themselves. Green leaves make food from Carbon dioxide and water in the presence of sunlight and chlorophyll. Hence, for taking place of photosynthesis carbon dioxide, water and sunlight must be reached at the green leaves in addition to presence of chlorophyll.
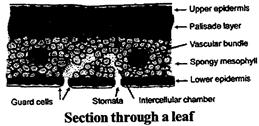 Leaves have several tiny pores more...
Leaves have several tiny pores more...  SOIL
The mixture of rock particles and humus is called soil. Soil is an important natural resource. It contains water, dissolved substances, mineral salts and living organisms. Soil forms a very thin layer on the surface of the earth ranging from a few m to 3 to 4 m.
Note: Humus is a brown or black organic substance formed from decaying plant remains or animal matter. It determines the fertility of soil. It is porous in nature and increases the ability of soil to retain water.
SOIL FORMATION
Soil is formed from parent rock material over millions of years by a process of weathering. Weathering is the process of breaking down of rock present on the surface of earth into fine particles.
Weathering Occurs by Two Main Processes
(a) Physical weathering, which is caused by physical phenomena like atmospheric changes (heating, cooling, wetting-drying etc).
(b) Biological weathering, which involves breaking down of rocks by the action of living organisms.
Do you know?
Earthworm’s burrows act as tunnels which allow water to moves quickly and provide pathways for roots to grow. They also decompose dead plants and animal matter. Their castings are valuable as fertilizer.
SOIL
The mixture of rock particles and humus is called soil. Soil is an important natural resource. It contains water, dissolved substances, mineral salts and living organisms. Soil forms a very thin layer on the surface of the earth ranging from a few m to 3 to 4 m.
Note: Humus is a brown or black organic substance formed from decaying plant remains or animal matter. It determines the fertility of soil. It is porous in nature and increases the ability of soil to retain water.
SOIL FORMATION
Soil is formed from parent rock material over millions of years by a process of weathering. Weathering is the process of breaking down of rock present on the surface of earth into fine particles.
Weathering Occurs by Two Main Processes
(a) Physical weathering, which is caused by physical phenomena like atmospheric changes (heating, cooling, wetting-drying etc).
(b) Biological weathering, which involves breaking down of rocks by the action of living organisms.
Do you know?
Earthworm’s burrows act as tunnels which allow water to moves quickly and provide pathways for roots to grow. They also decompose dead plants and animal matter. Their castings are valuable as fertilizer.
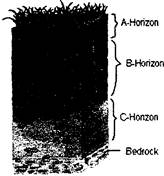 SOIL PROFILES
Soil profile is a vertical section of different layers of the soil. Various layers are called horizons. Each layer diners in colour, depth, chemical composition. Generally we see the top surface of the soil, not layers below it. Soil profile can be seen while digging a we'l or laying the foundation of a building. Soil profile i.e. various layers of soil can also be observed in a deep cut through the soil. Typically, four distinct soil layers can be seen. It can also be seen at the sides of a mad on a hill or at steep river bank.
A-Horizon
more...
SOIL PROFILES
Soil profile is a vertical section of different layers of the soil. Various layers are called horizons. Each layer diners in colour, depth, chemical composition. Generally we see the top surface of the soil, not layers below it. Soil profile can be seen while digging a we'l or laying the foundation of a building. Soil profile i.e. various layers of soil can also be observed in a deep cut through the soil. Typically, four distinct soil layers can be seen. It can also be seen at the sides of a mad on a hill or at steep river bank.
A-Horizon
more...  PHYSICAL PROPERTIES
The properties which describe the look or feel of a substance e.g., colour, hardness, density, texture and phase of matter, etc. Every substance has its own characteristic physical properties that we use to identify the substance.
PHYSICAL CHANGES
A change in which physical properties of substance such as, size, appearance or state, may alter, but its chemical composition remains the same and no new substance is formed are called physical changes. A physical change is a reversible change. For example dissolving sugar in water, cutting of wood etc.
Characteristics of Physical Changes
PHYSICAL PROPERTIES
The properties which describe the look or feel of a substance e.g., colour, hardness, density, texture and phase of matter, etc. Every substance has its own characteristic physical properties that we use to identify the substance.
PHYSICAL CHANGES
A change in which physical properties of substance such as, size, appearance or state, may alter, but its chemical composition remains the same and no new substance is formed are called physical changes. A physical change is a reversible change. For example dissolving sugar in water, cutting of wood etc.
Characteristics of Physical Changes
 Note: A mixture consist of two or more substances simply mixed together but not chemically combined. For example air is a mixture of various gases.
Note: A mixture consist of two or more substances simply mixed together but not chemically combined. For example air is a mixture of various gases.
 Note:- Although all acids taste sour and all bases taste bitter, a taste test is not the best general-purpose way to determine whether a substance is an acid or a base. Some acids and bases are poisonous, and some are quite corrosive. Never taste laboratory chemicals. Too many of them are toxic, and others might be contaminated.
ACIDS
Taste of substances containing acids is sour e.g. curd, lemon juice, orange juice, vinegar, etc. The acids in these substances are natural acids. Lemonade contains citric acid, grapes and tamarind contains tartaric acid. Synthetic substance such as vinegar contains acetic acid and cold drinks contain carbonic acid. The chemical nature of acids is acidic. The word acid comes from Latin word acere which means sour.
Do you know?
Robert boyle was the first to study the properties of acids and described them as sour, corrosive and turning blue litmus red.
Note:- Although all acids taste sour and all bases taste bitter, a taste test is not the best general-purpose way to determine whether a substance is an acid or a base. Some acids and bases are poisonous, and some are quite corrosive. Never taste laboratory chemicals. Too many of them are toxic, and others might be contaminated.
ACIDS
Taste of substances containing acids is sour e.g. curd, lemon juice, orange juice, vinegar, etc. The acids in these substances are natural acids. Lemonade contains citric acid, grapes and tamarind contains tartaric acid. Synthetic substance such as vinegar contains acetic acid and cold drinks contain carbonic acid. The chemical nature of acids is acidic. The word acid comes from Latin word acere which means sour.
Do you know?
Robert boyle was the first to study the properties of acids and described them as sour, corrosive and turning blue litmus red.
 Type of Acids
Acids are of two types
Type of Acids
Acids are of two types
 Do you know?
Sulphuric acid is known as the king of chemicals.
Do you know?
The earlier name for sulphuric acid was oil of vitriol due more...
Do you know?
Sulphuric acid is known as the king of chemicals.
Do you know?
The earlier name for sulphuric acid was oil of vitriol due more...  Do you know?
Natural fibres of animal origin are mostly protein fibres while those of plant source like cotton and jute are starch based.
WOOL
Wool is obtained from fleece of sheep and goat .It is also obtained from llama, alpaca, angora rabbit, yak etc. It is used in various ways to make woolen clothing, carpets, rugs and shelters.
Do you know?
Natural fibres of animal origin are mostly protein fibres while those of plant source like cotton and jute are starch based.
WOOL
Wool is obtained from fleece of sheep and goat .It is also obtained from llama, alpaca, angora rabbit, yak etc. It is used in various ways to make woolen clothing, carpets, rugs and shelters.
 Do you know?
Australia is the biggest grower of wool in the world.
Characteristics of Wool
Do you know?
Australia is the biggest grower of wool in the world.
Characteristics of Wool
 Do you know?
It is thought that wind energy was first used to propel sailing boats.
The air in certain regions becomes hot due to the heat from the sun. The hot air is lighter and so it rises up. As a result there is a drop of air pressure in that region. The cooler air from the colder regions being heavier starts flowing towards the low pressure regions. This air moving from the high pressure regions to the low pressure regions is called wind.
Do you know?
The movements of air toward the equator are called trade wind.
Air Pressure
Earth is surrounded by an envelope of air. The force exerted by air per unit surface area is called air pressure. If a can half-filled with water is heated till all the air is expelled out, and then covered with a lid. Thereafter cold water is poured over the can then it is found that the can gets crushed. It happens because on pouring cold water, the steam convert into water, thereby, reducing the pressure inside the can. The large air pressure outside the can crushes it. Air pressure is measured by barometer.
It is important to note that air pressure is exerted in all directions.
Consequences of Air Pressure
Do you know?
It is thought that wind energy was first used to propel sailing boats.
The air in certain regions becomes hot due to the heat from the sun. The hot air is lighter and so it rises up. As a result there is a drop of air pressure in that region. The cooler air from the colder regions being heavier starts flowing towards the low pressure regions. This air moving from the high pressure regions to the low pressure regions is called wind.
Do you know?
The movements of air toward the equator are called trade wind.
Air Pressure
Earth is surrounded by an envelope of air. The force exerted by air per unit surface area is called air pressure. If a can half-filled with water is heated till all the air is expelled out, and then covered with a lid. Thereafter cold water is poured over the can then it is found that the can gets crushed. It happens because on pouring cold water, the steam convert into water, thereby, reducing the pressure inside the can. The large air pressure outside the can crushes it. Air pressure is measured by barometer.
It is important to note that air pressure is exerted in all directions.
Consequences of Air Pressure
You need to login to perform this action.
You will be redirected in
3 sec
